Abstract
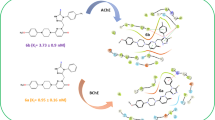
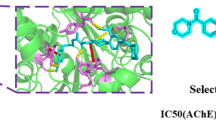
A series of 1,3,4-oxadiazole derivatives were designed, synthesized, and evaluated as acetylcholinesterase inhibitors. Their structure was confirmed by IR, NMR, and high-resolution mass spectra. The synthesized compounds showed significant acetylcholinesterase inhibitory activity with an IC50 value of 0.07 μM for the most potent compound. Molecular docking study of the most active compound indicated that it interacted with the crucial amino acids present at the catalytic active site and peripheral anionic site of acetylcholinesterase. The results suggested that the obtained 1,3,4-oxadiazole derivatives may be the potential drug candidates for the treatment of Alzheimer’s disease as AChE inhibitors.



Similar content being viewed by others
REFERENCES
De Bruijn, R.F. and Ikram, M.A., BMC Med., 2014, vol. 12, p. 130. https://doi.org/10.1186/s12916-014-0130-5
Lasagna‐Reeves, C.A., Castillo‐Carranza, D.L., Sengupta, U., Sarmiento, J., Troncoso, J., Jackson, G.R., and Kayed, R., FASEB J., 2012, vol. 26, p. 1946. https://doi.org/10.1096/fj.11-199851
Yang, S., Kaoru, Y., Shane, A.L., Scott, T.S., Zhao, Z., Luo, W., Richard, M.T., Salvatore, S., Lea, T., and Julio, C.R., Nature, 2017, vol. 549, p. 523. https://doi.org/10.1038/nature24016
Choubey, P.K., Tripathi, A., Tripathi, M.K., Seth, A., and Shrivastava, S.K., Bioorg. Chem., 2021, vol. 111, article ID 104922. https://doi.org/10.1016/j.bioorg.2021.104922
Parri, H.R., Hernandez, C.M., and Dineley, K.T., Biochem. Pharmacol., 2011, vol. 82, p. 931. https://doi.org/10.1016/j.bcp.2011.06.039
Lakshmithendral, K., Saravanan, K., Elancheran, R., Archana, K., Manikandan, N., Arjun, H.A., Ramanathan, M., Lokanath, N.K., and Kabilan, S., Eur. J. Med. Chem., 2019, vol. 168, p. 1. https://doi.org/10.1016/j.ejmech.2019.02.033
Sekhar, M.M., Nagarjuna, U., Padmavathi, V., Padmaja, A., Reddy, N.V., and Vijaya, T., Eur. J. Med. Chem., 2018, vol. 145, p. 1. https://doi.org/10.1016/j.ejmech.2017.12.067
Dewangan, D., Verma, V.S., Nakhate, L.T., Tripathi, D.K., Kashyap, P., and Dhongade, H., Med. Chem. Res., 2016, vol. 25, p. 2143. https://doi.org/10.1007/s00044-016-1641-8
Chaves, J.D.S., Tunes, L.G., Franco, C.H.J., Francisco, T.M., Corrêa, C.C., and Silvane, M.F., Eur. J. Med. Chem., 2017, vol. 127, p. 727. https://doi.org/10.1016/j.ejmech.2016.10.052
El-Sayed, W.A., El-Essawy, F.A., Ali, O.M., Nasr, B.S., Abdalla, M.M., and Abdel-Rahman, A.A.-H., Z. Naturforsch., Teil C, 2009, vol. 64, p. 773. https://doi.org/10.1515/znc-2009-11-1203
Mochona, B., Mazzio, E., Gangapurum, M., Mateeva, N., and Redda, K., Chem. Sci. Trans., 2015, vol. 4, p. 534. https://doi.org/10.7598/cst2015.1029
Ahmed, K, Shaik, A.B., Reddy, G.N, Kumar, C.G., Joseph, J., Kumar, G.B., Purushotham, U., and Sastry, G.N., Med. Chem. Res., 2014, vol. 23, p. 2080. https://doi.org/10.1007/s00044-013-0786-y
Mei, W.-W., Ji, Sh.-Sh., Xiao, W., Wang, X.-D., Jiang, Ch.-Sh., Ma, W.-Q., Zhang, H.-Y., Gong, J.-X., and Guo, Y.-W., Monatsh. Chem., 2017, vol. 148, p. 1807. https://doi.org/10.1007/s00706-017-1993-x
Tripathi, A., Choubey, P.K., Sharma, P., Seth, A., Tripathi, P.N., Tripathi, M.K., Prajapati, S.K., Krishnamurthy, S., and Shrivastava, S.K., Eur. J. Med. Chem., 2019, vol. 183, article ID 111707. https://doi.org/10.1016/j.ejmech.2019.111707
Banday, M.R., Mattoo, R.H., and Rauf, A., J. Chem. Sci., 2010, vol. 122, p. 177. https://doi.org/10.1007/s12039-010-0019-6
Wang, S., Qi, L., Liu, H., Lei, K., and Liu, R., RSC Adv., 2020, vol. 10, p. 30848. https://doi.org/10.1039/D0RA05886F
Shi, J., Luo, N., Ding, M., and Bao, X., Chin. Chem. Lett., 2020, vol. 31, p. 434. https://doi.org/10.1016/j.cclet.2019.06.037
Ellman, G., Courtney, K.D., Andres Jr, V., and Featherstone, R.M., Biochem. Pharmacol., 1961, vol. 7, p. 88. https://doi.org/10.1016/0006-2952(61)90145-9
Song, M.Q., Min, W., Wang, J., Si, X.X., Wang, X.J., Liu, Y.W., and Shi, D.H., J. Mol. Struct., 2021, vol. 1229, article ID 129784. https://doi.org/10.1016/j.molstruc.2020.129784
Funding
The authors gratefully acknowledge financial supports of Graduate Research and Innovation Projects of Jiangsu Province (KYCX20-2895), Natural Science Foundation of Jiangsu Province (BK20191470), and project funded by the Priority Academic Program Development of Jiangsu higher education institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, S., Zou, JP., Li, XR. et al. Synthesis and Biological Evaluation of 1,3,4-Oxadiazole Derivatives as Acetylcholinesterase Inhibitors. Russ J Org Chem 58, 1520–1526 (2022). https://doi.org/10.1134/S1070428022100207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022100207