Abstract
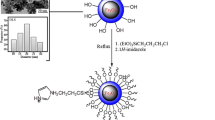
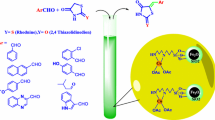
1H-Indazolo[2,1-b]phthalazine-1,6,11-trione derivatives have been synthesized through a one-pot three-component condensation of phthalhydrazide, dimedone, and aromatic aldehydes at 80°C under solvent-free conditions in the presence of a novel Co nanoparticle catalyst. The catalyst was prepared by coating Fe3O4 magnetic nanoparticles with tetraethyl orthosilicate, followed by functionalization with 3-chloropropyl(trimethoxy)silane and 2-amino-1,3,4-thiadiazole-5(4H)-thione and complexation with cobalt(II) acetate. The superparamagnetic catalyst could be readily recovered by applying an external super magnet and used for several times without significant loss of catalytic activity.




Similar content being viewed by others
REFERENCES
Mourad, A.K., Makhlouf, A.A., and Soliman, A.Y., J. Chem. Res., 2019, vol. 44, p. 31. https://doi.org/10.1177/1747519819883840
Zhang, L., Guan, L-P., Sun, X-Y., Wei, C-X., Chai, K-Y., and Quan, Z-S., Chem. Biol. Drug Des., 2009, vol. 73, p. 313. https://doi.org/10.1111/j.1747-0285.2009.00776.x
Ryu, C.-K., Park, R.-E., Ma, M.-Y., and Nho, J.-H., Bioorg. Med. Chem. Lett., 2007, vol. 17, p. 2577. https://doi.org/10.1016/j.bmcl.2007.02.003
Li, J., Zhao, Y.-F., Yuan, X.-Y., Xu, J.-X., and Gong, P., Molecules, 2006, vol. 11, p. 574. https://doi.org/10.3390/11070574
Sinkkonen, J., Ovcharenko, V., Zelenin, K., Bezhan, I., Chakchir, B., Al-Assar, F., and Pihlaja, K., Eur. J. Org. Chem., 2002, vol. 2002, no. 13, p. 2046. https://doi.org/10.1002/1099-0690(200207)2002:13<2046::AID-EJOC2046>3.0.CO;2-C
Nomoto, Y., Obase, H., Takai, H., Teranishi, M., Nakamura, J., and Kubo, K., Chem. Pharm. Bull., 1990, vol. 38, p. 2179. https://doi.org/10.1248/cpb.38.2179
Watanabe, N., Kabasawa, Y., Takase, Y., Matsukura, M., Miyazaki, K., Ishihara, H., Kodama, K., and Adachi, H., J. Med. Chem., 1998, vol. 41, p. 3367. https://doi.org/10.1021/jm970815r
Ghahremanzadeh, R., Shakibaei, G.I., and Bazgir, A., Synlett, 2008, vol. 2008, p. 1129. https://doi.org/10.1055/s-2008-1072716
Ghahremanzadeh, R., Ahadi, S., Sayyafi, M., and Bazgir, A., Tetrahedron Lett., 2008, vol. 29, p. 4479. https://doi.org/10.1016/j.tetlet.2008.05.063
Grasso, S., De Sarro, G., De Sarro, A., Micale, N., Zappalà, M., Puja, G., Baraldi, M., and De Micheli, C., J. Med. Chem., 2000, vol. 43, p. 2851. https://doi.org/10.1021/jm001002x
Sheradsky, T. and Moshenberg, R., J. Org. Chem., 1986, vol. 51, p. 3123. https://doi.org/10.1021/jo00366a008
Heine, H.W., Baclawski, L.M., Bonser, S.M., and Wachob, G.D., J. Org. Chem., 1976, vol. 41, p. 3229. https://doi.org/10.1021/jo00882a002
Ramtohul, Y.K., James, M.N., and Vederas, J.C., J. Org. Chem., 2002, vol. 67, p. 3169. https://doi.org/10.1021/jo0157831
Liu, L.-P., Lu, J.-M., and Shi, M., Org. Lett., 2007, vol. 9, p. 1303. https://doi.org/10.1021/ol070178r
CsXmpai, A., Körmendy, K., and Ruff, F., Tetrahedron, 1991, vol. 47, p. 4457. https://doi.org/10.1016/S0040-4020(01)87114-3
Amarasekara, A.S. and Chandrasekara, S., Org. Lett., 2002, vol., 4, p. 773. https://doi.org/10.1021/ol017256+
Hwang, J.Y., Choi, H.-S., and Gong, Y.-D., Tetrahedron Lett., 2005, vol. 46, p. 3107. https://doi.org/10.1016/j.tetlet.2005.02.154
Quiroga, J., Mejı́a, D., Insuasty, B., Abonı́a, R., Nogueras, M., Sánchez, A., Cobo, J., and Low, J.N., Tetrahedron, 2001, vol. 57, p. 6947. https://doi.org/10.1016/S0040-4020(01)00649-4
Quiroga, J., Hormaza, A., Insuasty, B., Ortiz, A., Sánchez, A., and Nogueras, M., J. Heterocycl. Chem., 1998, vol. 35, p. 231. https://doi.org/10.1002/jhet.5570350142
Tu, S., Fang, F., Li, T., Zhu, S., and Zhang, X., J. Heterocycl. Chem., 2005, vol. 42, p. 707. https://doi.org/10.1002/jhet.5570420436
Quiroga, J., Insuasty, B., Hormaza, A., Saitz, C., and Jullian, C., J. Heterocycl. Chem., 1998, vol. 35, p. 575. https://doi.org/10.1002/jhet.5570350313
Shaabani, A., Rahmati, A., and Naderi, S., Bioorg. Med. Chem. Lett., 2005, vol. 15, p. 5553. https://doi.org/10.1016/j.bmcl.2005.08.101
Wang, X., Ma, W.W., Wu, L.Q., and Yan, F.L., J. Chin. Chem. Soc., 2010, vol. 57, p. 1341. https://doi.org/10.1002/jccs.201000198
Shaterian, H.R., Hosseinian, A., and Ghashang, M., Arkivoc, 2009, vol. 2009, no. 2, p. 59. https://doi.org/10.3998/ark.5550190.0010.207
Shekouhy, M. and Hasaninejad, A., Ultrason. Sonochem., 2012, vol. 19, p. 307. https://doi.org/10.1016/j.ultsonch.2011.07.011
Sarrafioun, F., Jamehbozorgi, S., and Ramezani, M., Russ. J. Org. Chem., 2019, vol. 55, p. 1777. https://doi.org/10.1134/S1070428019110216
ACKNOWLEDGMENTS
The authors are grateful to the Islamic Azad University (Faculty of Sciences, Arak Branch, Arak Iran) and Islamic Azad University (Faculty of Sciences, Hamadan Branch, Hamadan, Iran) for the technical support of this study. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Sarrafioun, F., Jamehbozorgi, S., Ramezani, M. et al. Synthesis of Phthalazine Derivatives through a One-Pot Three-Component Reaction Using a Highly Efficient and Recyclable Magnetic Cobalt Nanocatalyst. Russ J Org Chem 58, 1481–1486 (2022). https://doi.org/10.1134/S1070428022100141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022100141