Abstract
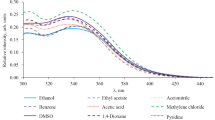
Previously unknown 3,4-diamino-6-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles were synthesized by reaction of 4-amino-6-aryl-2-bromopyridine-3,5-dicarbonitriles with hydrazine hydrate. Study of the optical properties of the synthesized compounds revealed their fluorescence in solution with the emission maximum located in the range of λ 484–548 nm and quantum yield of 0.9–3.9%.


Similar content being viewed by others
REFERENCES
Ye, Q., Cao, J., Zhou, X., Lv, D., He, Q., Yang, B., and Hu, Y., Bioorg. Med. Chem., 2009, vol. 17, p. 4763. https://doi.org/10.1016/j.bmc.2009.04.043
Witherington, J., Bordas, V., Garland, S.L., Hickey, D.M.B., Ife, R.J., Liddle, J., Saunders, M., Smith, D.G., and Ward, R.W., Bioorg. Med. Chem. Lett., 2003, vol. 13, p. 1577. https://doi.org/10.1016/S0960-894X(03)00134-3
Nagender, P., Malla Reddy, G., Naresh Kumar, R., Poornachandra, Y., Ganesh Kumar, C., and Narsaiah, B., Bioorg. Med. Chem. Lett., 2014, vol. 24, p. 2905. https://doi.org/10.1016/j.bmcl.2014.04.084
Mohamed, M.S., Awad, Y.E.E.-D., El-Hallouty, S.M., and El-Araby, M., Open J. Med. Chem., 2012, vol. 2, p. 78. https://doi.org/10.4236/ojmc.2012.23010
Ravi Kumar, N., Poornachandra, Y., Krishna Swaroop, D., Jitender Dev, G., Ganesh Kumar, C., and Narsaiah, B., Bioorg. Med. Chem. Lett., 2016, vol. 26, p. 5203. https://doi.org/10.1016/j.bmcl.2016.09.062
Hao, S.-Y., Qi, Z.-Y., Wang, S., Wang, X.-R., and Chen, S.-W., Bioorg. Med. Chem., 2021, vol. 31, article ID 115985. https://doi.org/10.1016/j.bmc.2020.115985
Ribeiro, J.L.S., Soares, J.C.A.V., Portapilla, G.B., Providello, M.V., Lima, C.H.S., Muri, E.M.F., de Albuquerque, S., and Dias, L.R.S., Bioorg. Med. Chem., 2021, vol. 29, article ID 115855. https://doi.org/10.1016/j.bmc.2020.115855
Umar, T., Shalini, S., Raza, M.K., Gusain, S., Kumar, J., Seth, P., Tiwari, M., and Hoda, N., Eur. J. Med. Chem., 2019, vol. 175, p. 2. https://doi.org/10.1016/j.ejmech.2019.04.038
Huart, A.-S., Saxty, B., Merritt, A., Nekulova, M., Lewis, S., Huang, Y., Vojtesek, B., Kettleborough, C., and Hupp, T.R., Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 5578. https://doi.org/10.1016/j.bmcl.2013.08.046
Shi, J., Xu, G., Zhu, W., Ye, H., Yang, S., Luo, Y., Han, J., Yang, J., Li, R., Wei, Y., and Chen, L., Bioorg. Med. Chem. Lett., 2010, vol. 20, p. 4273. https://doi.org/10.1016/j.bmcl.2010.04.083
Zhao, B., Li, Y., Xu, P., Dai, Y., Luo, C., Sun, Y., Ai, J., Geng, M., and Duan, W., ACS Med. Chem. Lett., 2016, vol. 7, p. 629. https://doi.org/10.1021/acsmedchemlett.6b00066
Chen, J., Liu, W., Ma, J., Xu, H., Wu, J., Tang, X., Fan, Z., and Wang, P., J. Org. Chem., 2012, vol. 77, p. 3475. https://doi.org/10.1021/jo3002722
Deore, R., Dingore, K., and Jachak, M., J. Fluoresc., 2015, vol. 25, p. 1549. https://doi.org/10.1007/s10895-015-1674-2
Kendre, D.B., Toche, R.B., and Jachak, M.N., Tetrahedron, 2007, vol. 63, p. 11000. https://doi.org/10.1016/j.tet.2007.08.052
García, M., Romero, I., and Portilla, J., ACS Omega, 2019, vol. 4, p. 6757. https://doi.org/10.1021/acsomega.9b00226
Orrego-Hernández, J., Lizarazo, C., Cobo, J., and Portilla, J., RSC Adv., 2019, vol. 9, p. 27318. https://doi.org/10.1039/C9RA04682H
Sirakanyan, S.N., Ghazaryan, S.G., Hakobyan, E.K., and Hovakimyan, A.A., Russ. J. Org. Chem., 2020, vol. 56, p. 840. https://doi.org/10.1134/S1070428020050176
Sirakanyan, S.N., Hakobyan, E.K., and Hovakimyan, A.A., Russ. J. Org. Chem., 2018, vol. 54, p. 929. https://doi.org/10.1134/S1070428018060167
Hamza, E.Kh., Hamdy, N.A., Zarie, E.S., Fakhr, I.M.I., Elwahy, A.H.M., and Awad, H.M., J. Heterocycl. Chem., 2019, vol. 57, p. 182. https://doi.org/10.1002/jhet.3764
Sanad, S.M.H., Hawass, M.A.E., Ahmed, A.A.M., and Elneairy, M.A.A., Synth. Commun., 2018, vol. 48, p. 1847. https://doi.org/10.1080/00397911.2018.1468911
Sanad, S.M.H., Abdel-Fattah, A.M., Attaby, F.A., and Elneairy, M.A.A., J. Heterocycl. Chem., 2018, vol. 56, p. 651. https://doi.org/10.1002/jhet.3444
Abdel Fattah, A.M., Elneairy, M.A.A., and Gad-Elkareem, M.A.M., Phosphorus, Sulfur Silicon Relat. Elem., 2007, vol. 182, p. 1351. https://doi.org/10.1080/10426500601160991
Bardasov, I.N., Alekseeva, A.U., and Ershov, O.V., Tetrahedron Lett., 2018, vol. 59, p. 1398. https://doi.org/10.1016/j.tetlet.2018.02.069
Bardasov, I.N., Mihailov, D.L., Alekseeva, A.U., Ershov, O.V., and Nasakin, O.E., Tetrahedron Lett., 2013, vol. 54, p. 21. https://doi.org/10.1016/j.tetlet.2012.10.015
Ershov, O.V., Mikhailov, D.L., Bardasov, I.N., Ievlev, M.Yu., and Belikov, M.Yu., Russ. J. Org. Chem., 2017, vol. 53, p. 886. https://doi.org/10.1134/S1070428017060124
Ershov, O.V., Ievlev, M.Yu., Belikov, M.Yu., and Maksimova, V.N., Russ. J. Org. Chem., 2018, vol. 54, p. 873. https://doi.org/10.1134/S1070428018060088
Tranfić, M., Halambek, J., Cetina, M., and Jukić, M., J. Mol. Struct., 2011, vol. 1001, p. 145. https://doi.org/10.1016/j.molstruc.2011.06.033
El-Sayed, A.A., Amr, A.E., El-Ziaty, A.K., and Elsayed, E.A., Molecules, 2019, vol. 24, article no. 1965. https://doi.org/10.3390/molecules24101965
ACKNOWLEDGMENTS
A part of this study was performed in the framework of state assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental research.
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (project no. 0849-2020-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2022, Vol. 58, No. 7, pp. 754–759 https://doi.org/10.31857/S0514749222070084.
Rights and permissions
About this article
Cite this article
Al-Shuaeeb, R.A.A., Alekseeva, A.Y., Yashchenko, N.N. et al. Synthesis and Optical Properties of 3,4-Diamino-6-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles. Russ J Org Chem 58, 997–1001 (2022). https://doi.org/10.1134/S1070428022070089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022070089