Abstract
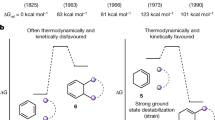
All possible transition state structures that could mediate thermally induced skeletal transformations of cyclooctatetraene in an oxygen-free atmosphere have been identified on the basis of the properties of the π-conjugated system in cyclic polyenes. DFT study of the potential energy surface and the nature of localized stationary points using the B3LYP functional and 6-31G* basis set confirmed the existence of 30 transition states. A scheme for the thermal isomerization of cyclooctatetraene, which includes 47 forward and reverse reactions, was constructed by the Gonzalez–Schlegel method for the determination of potential barriers. The obtained results were consistent with the available experimental data.








Similar content being viewed by others
REFERENCES
Denisov, E.T., Russ. Chem. Rev., 2000, vol. 69, p. 166. https://doi.org/10.1070/RC2000v069n02ABEH000560
Müller, T. and Mingos, D., Transit. Met. Chem., 1995, vol. 20, p. 533. https://doi.org/10.1007/BF00136415
Serezhkin, V.N., Pushkin, D.V., Serezhkina, L.B., Sevast’yanov, V.G., and Kuznetsov, N.T., Zh. Neorg. Khim., 2005, vol. 50, p. 2019.
Tomilin, O.B., Tanaseichuk, B.S., and Boyarkina, O.V., Russ. J. Org. Chem., 2016, vol. 52, p. 1576. https://doi.org/10.1134/S1070428016110051
Rodionova, E.V., Tomilin, O.B., and Fomina, L.V., Russ. J. Org. Chem., 2021, vol. 57, p. 135. https://doi.org/10.1134/S1070428021020019
Scott, L.T. and Jones, M., Chem. Rev., 1972, vol. 72, p. 181. https://doi.org/10.1021/cr60276a004
Andrés, J.L., Castaño, O., Morreale, A., Palmeiro, R., and Gomperts, R., Chem. Phys., 1998, vol. 108, p. 203. https://doi.org/10.1063/1.475388
Garavelli, M., Bernardi, F., Cembran, A., Castano, O., Frutos, L.M., Merchan, M., and Olivucci, M., J. Am. Chem. Soc., 2002, vol. 124, p. 13770. https://doi.org/10.1021/ja020741v
Klärner, F.G., Angew. Chem., Int. Ed., 2001, vol. 40, p. 3977. https://doi.org/10.1002/1521-3773
Deslongchamps, G. and Deslongchamps, P., J. Org. Chem., 2018, vol. 83, p. 5751. https://doi.org/10.1021/acs.joc.8b00809
Wu, J.I., Fernandez, I., Mo, Y., and Schleyer, P.v.R., J. Chem. Theory Comput., 2012, vol. 8, p. 1280. https://doi.org/10.1021/ct3000553
Nishinaga, T., Ohmae, T., and Iyoda, M., Symmetry, 2010, vol. 2, p. 76. https://doi.org/10.3390/sym2010076
Hassenrück, K., Martin, H.-D., and Walsh, R., Chem. Rev., 1989, vol. 89, p. 1125. https://doi.org/10.1021/cr00095a010
Jiao, H.J., Nagelkerke, R., Kurtz, H.A., Williams, R.V., Borden, W.T., and Schleyer, P.v.R., J. Am. Chem. Soc., 1997, vol. 119, p. 5921. https://doi.org/10.1021/ja963165+
Rücker, C. and Prinzbach, H., Angew. Chem., Int. Ed., 1985, vol. 24, p. 411. https://doi.org/10.1002/anie.198504111
Christl, M., Lang, R., and Herzog, C., Tetrahedron, 1986, vol. 42, p. 1585. https://doi.org/10.1016/S0040-4020(01)87575-X
Smith, L.R., Gream, G.E., and Meinwald, J., J. Org. Chem., 1977, vol. 42, p. 927. https://doi.org/10.1021/jo00426a001
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguyen, K.A., Su, S., Windus, T.L., Dupuis, M., and Montgomery, J.A., J. Comput. Chem., 1993, vol. 14, p. 1347. https://doi.org/10.1002/jcc.540141112
Gonzalez, C. and Schlegel, H.B., J. Phys. Chem., 1990, vol. 94, p. 5523. https://doi.org/10.1021/j100377a021
Seyferth, D., Organometallics, 2004, vol. 23, p. 3562. https://doi.org/10.1021/om0400705
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2022, Vol. 58, No. 4, pp. 392–405 https://doi.org/10.31857/S0514749222040048.
Rights and permissions
About this article
Cite this article
Tomilin, O.B., Fomina, L.V. & Rodionova, E.V. Possible Skeletal Transformations of Cyclooctatetraene in Its Thermal Isomerization. Russ J Org Chem 58, 488–498 (2022). https://doi.org/10.1134/S1070428022040042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022040042