Abstract
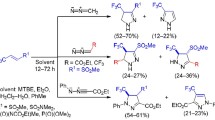
Electron-deficient conjugated enynes reacted with diazomethane under mild conditions at the C=C double bond to give the corresponding nitrogen-containing cycloadducts or cyclopropanes. The product structure depended on the number of electron-withdrawing groups at the double bond of the initial enyne. 1,5-Diarylpent-2-en-4-yn-1-ones were converted to 4,5-dihydro-1H-pyrazoles, propargylidenemalonic acid derivatives gave rise to 4,5-dihydro-3H-pyrazoles, and 5,7-dioxaspiro[2.5]octane-4,8-diones were formed from enyne derivatives of Meldrum’s acid. Noncatalytic methods were developed for the synthesis of spirocyclic cyclopropanes and ethynyl-substituted dihydropyrazoles in 75–90% yields.





Similar content being viewed by others
REFERENCES
Breugst, M., Huisgen, R., and Reissig, H.-U., Eur. J. Org. Chem., 2018, vol. 2018, p. 2477. https://doi.org/10.1002/ejoc.201800100
Menchikov, L.G., Shulishov, E.V., and Tomilov, Yu.V., Russ. Chem. Rev., 2021, vol. 90, p. 199. https://doi.org/10.1070/RCR4982
Latypova, D.R., Dokichev, V.A., and Zlotskii, S.S., Uspekhi khimii diazosoedinenii. Metody polucheniya, reaktsii, svoistva (Advances in the Chemistry of Diazo Compounds. Methods of Synthesis, Reactions, and Properties), Saarbryukken: Lambert Academic Publishing, 2012, p. 7.
Yang, H., Martin, B., and Shenkel, B., Org. Process Res. Dev., 2018, vol. 22, p. 446. https://doi.org/10.1021/acs.oprd.7b00302
Proctor, L.D. and Warr, A.J., Org. Process Res. Dev., 2002, vol. 6, p. 884. https://doi.org/10.1021/op020049k
Bennani, F.E., Doudach, L., Cherrah, Y., Ramli, Y., Karrouchi, K., Ansar, M., and Faouzi, M.E.A., Bioorg. Chem., 2020, vol. 97, article ID 103470. https://doi.org/10.1016/j.bioorg.2019.103470
Salaün, J., Small Ring Compounds in Organic Synthesis VI, de Meijere, A., Ed., 2000, p. 1. https://doi.org/10.1007/3-540-48255-5
Korobitsyna, I.K., Bulusheva, V.V., and Rodina, L.L., Chem. Heterocycl. Compd., 1978, vol. 14, p. 471. https://doi.org/10.1007/BF00673325
Bettinetti, G., Desimoni, G., and Grunanger, P., Gazz. Chem. Ital., 1964, vol. 94, p. 91.
Vo-Quang, L. and Vo-Quang, Y., Bull. Soc. Chim. Fr., 1974, p. 2575.
Reimlinger, H. and Moussebois, C.H., Chem. Ber., 1965, vol. 98, p. 1805. https://doi.org/10.1002/cber.19650980619
Yangirov, T.A., Cand. Sci. (Chem.) Dissertation, Ufa. 2013.
Golovanov, A.A., Odin, I.S., and Zlotskii, S.S., Russ. Chem. Rev., 2019, vol. 88, p. 280. https://doi.org/10.1070/RCR4808
Golovanov, A.A., Gusev, D.M., Odin, I.S., and Zlotskii, S.S., Chem. Heterocycl. Compd., 2019, vol. 55, p. 333. https://doi.org/10.1007/s10593-019-02462-0
Odin, I.S., Golovanov, A.A., Bekin, V.V., and Pisareva, V.S., Chem. Heterocycl. Compd., 2014, vol. 49, p. 1687. https://doi.org/10.1007/s10593-014-1421-7
Sokov, S.A., Odin, I.S., Gusev, D.M., Kunavin, Yu.A., Vologzhanina, A.V., Voronova, E.D., and Golovanov, A.A., Russ. Chem. Bull., Int. Ed., 2020, vol. 69, p. 305. https://doi.org/10.1007/211172-020-2761-3
Maurya, R.A., Kapure, J.S., Adiyala, P.R., Srikanth, P.S., Chandrasekhar, D., and Kamal, A., RSC Adv., 2013, vol. 3, p. 15600. https://doi.org/10.1039/C3RA42374C
Klimova, V.A., Osnovnye mikrometody analiza organicheskikh soedinenii (Main Micro Methods for Analysis of Organic Compounds), Moscow: Khimiya, 1975, p. 51.
Shulishov, E.V., Klimenko, I.P., and Tomilov, Yu.V., Sintezy organicheskikh soedinenii (Syntheses of Organic Compounds), Moscow: Maks Press, 2008, vol. 3.
Golovanov, A.A., Latypova, D.R., Bekin, V.V., Pisareva, V.S., Vologzhanina, A.V., and Dokichev, V.A., Russ. J. Org. Chem., 2013, vol. 49, p. 1264. https://doi.org/10.1134/S1070428013090030
Sokov, S.A., Odin, I.S., Zlotskii, S.S., and Golovanov, A.A., Russ. J. Org. Chem., 2020, vol. 56, p. 1758. https://doi.org/10.1134/S1070428020100140
Saulnier, S., Golovanov, A.A., Ivanov, A.Yu., Boyarskaya, I.A., and Vasilyev, A.V., J. Org. Chem., 2016, vol. 81, p. 1967. https://doi.org/10.1021/acs.joc.5b02785
Golovanov, A.A., Dan’kov, S.A., Sokov, S.A., Melnikov, P.A., Ukolov, A.I., Voronova, E.D., Vologzhanina, A.V., and Bunev, A.S., Chem. Heterocycl. Compd., 2019, vol. 55, p. 93. https://doi.org/10.1007/s10593-019-02424-6
Belil, C., Pascual, J., and Serratosa, F., Tetrahedron, 1964, vol. 20, p. 2701. https://doi.org/10.1016/S0040-4020(01)90851-8
ACKNOWLEDGMENTS
The authors thank K.V. Gordon for performing elemental analyses.
Funding
This study was performed under financial support by the Leader Project Competition of the Ufa State Petroleum Technological University (nomination “Scientific Research Foundation”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 10, pp. 1371–1381 https://doi.org/10.31857/S0514749221100025.
Rights and permissions
About this article
Cite this article
Sokov, S.A., Odin, I.S., Zlotski, S.S. et al. Reactions of Activated Enynes with Diazomethane. Russ J Org Chem 57, 1575–1583 (2021). https://doi.org/10.1134/S107042802110002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042802110002X