Abstract
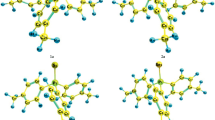
The polarity and structure of tri(1- or 2-naphthyl)phosphines and their chalcogenides were determined by the methods of dipole moments, IR spectroscopy, and DFT quantum-chemical calculations at the B3PW91/6-311++G(df,p) level of theory. In solution, tri(1-naphthyl)phosphine prefers a single conformer with a gauche,gauche,gauche orientation of the substituents at the phosphorus. Tri(2-naphthyl)phosphine, as well as both phosphine chalcogenides exist as equilibrium mixtures of several forms with a propeller arrangement of the substituents and a cis or gauche orientation of the Csp2‒Csp2 and P=X (X = LEP, O, S, Se) bonds.






Similar content being viewed by others
REFERENCES
Dabbawala, A.A., Bajaj, H.C., Rao, G.V.S., and Abdi, S.H.R., Appl. Catal. A: Gen., 2012, vol. 419, p. 185. https://doi.org/10.1016/j.apcata.2012.01.027
Artem’ev, A.V., Kuimov, V.A., Matveeva, E.A., Bagryanskaya, I.Yu., Govdi, A.I., Vasilevsky, S.F., Rakhmanova, M.I., Samultsev, D.O., Gusarova, N.K., and Trofimov, B.A., Inorg. Chem. Commun., 2017, vol. 86, p. 94. https://doi.org/10.1016/j.inoche.2017.09.008
Qin, C., Wu, H., Cheng, J., Chen, X., Liu, M., Zhang, W., Su, W., and Ding, J., J. Org. Chem., 2007, vol. 72, p. 4102. https://doi.org/10.1021/jo070267h
Zhao, H., Cheng, M., Zhang, T., and Cai, M., J. Organomet. Chem., 2015, vol. 777, p. 50. https://doi.org/10.1016/j.jorganchem.2014.11.020
Desroches, J., Tremblay, A., and Paquin, J.-F., Org. Biomol. Chem., 2016, vol. 14, p. 8764. https://doi.org/10.1039/c6ob01663d
Hoen, R., Tiemersma-Wegman, T., Procuranti, B., Lefort, L., de Vries, J.G., Minnaard, A.J., and Feringa, B.L., Org. Biomol. Chem., 2007, vol. 5, p. 267. https://doi.org/10.1039/b615131k
Dabbawala, A.A., Jasra, R.V., and Bajaj, H.C., Catal. Commun., 2011, vol. 12, p. 403. https://doi.org/10.1016/j.catcom.2010.10.026
Onodera, G., Hachisuka, R., Noguchi, T., Miura, H., Hashimoto, T., and Takeuchi, R., Tetrahedron Lett., 2014, vol. 55, p. 310. https://doi.org/10.1016/j.tetlet.2013.10.085
Elard, M., Denis, J., Ferreira, M., Bricout, H., Landy, D., Tilloy, S., and Monflier, E., Catal. Today, 2015, vol. 247, p. 47. https://doi.org/10.1016/j.cattod.2014.06.002
de Aquino, A., Caparros, F.J., Aullon, G., Ward, J.S., Rissanen, K., Jung, Y., Choi, H., Lima, J.C., and Rodríguez, L., Chem. Eur. J., 2021, vol. 27, p. 1810. https://doi.org/10.1002/chem.202004051
Hobbollahi, E., List, M., Redhammer, G., Zabel, M., and Monkowius, U., Inorg. Chem. Commun., 2016, vol. 65, p. 24. https://doi.org/10.1016/j.inoche.2016.01.009
Svahn, N., Moro, A.J., Roma-Rodridues, C., Puttreddy, R., Rissanen, K., Baptista, P.V., Fernandes, A.R., Lima, J.C., and Rodriguez, L., Chem. Eur. J., 2018, vol. 24, p. 14654. https://doi.org/10.1002/chem.201802547
Arumugam, R., Shankar, B., Arumuganathan, T., and Sathiyendiran, M., J. Organomet. Chem., 2021, vol. 933, p. 121657. https://doi.org/10.1016/j.jorganchem.2020.121657
Davis, W.L. and Muller, A., Acta Crystallogr., Sect. E, 2012, vol. 68, p. o3484. https://doi.org/10.1107/S1600536812048234
Govdi, A.I., Vasilevsky, S.F., Malysheva, S.F., Kazheva, O.N., Dyachenko, O.A., and Kuimov, V.A., Heteroatom Chem., 2018, vol. 29, p. e21443. https://doi.org/10.1002/hc.21443
Meijboom, R., Acta Crystallogr., Sect. E, 2011, vol. 67, p. m1438. https://doi.org/10.1107/S1600536811038505
Ogutu, H. and Meijboom, R., Acta Crystallogr., Sect. E, 2012, vol. 68, p. m394. https://doi.org/10.1107/S1600536812008148
Ishmaeva, E.A., Timosheva, A.P., Timosheva, N.V., and Vereshchagina, Ya.A., Spravochnik po dipol’nym momentam fosfororganicheskikh soedinenii (Handbook on Dipol Momenr of Organophosphorous Compounds), Kazan: Izd. Kazan. Univ., 1998.
Vereshchagina, Y.A., Khanafieva, R.R., Chachkov, D.V., Ishmaeva, E.A., Malysheva, S.F., Gusarova, N.K., and Trofimov, B.A., Pure Appl. Chem., 2017, vol. 89, p. 393. https://doi.org/10.1515/pac-2016-0802
Muller, A., Acta Crystallogr., Sect. E, 2011, vol. 67, p. o45. https://doi.org/10.1107/S1600536810050567
Malysheva, S.F., Kuimov, V.A., Belogorlova, N.A., Albanov, A.I., Gusarova, N.K., and Trofimov, B.A., Eur. J. Org. Chem., 2019, p. 6240. https://doi.org/10.1002/ejoc.201901005
Minkin, V.I., Osipov, O.A., and Zhdanov, Yu.A., Dipol’nye momenty v organicheskoi khimii (Dipole Moments in Organic Chemistry), Leningrad: Khimiya, 1968.
Gribov, L.A. and Popov, E.M., Doklady Akad. Nauk SSSR, 1962, vol. 145, p. 761.
Lux, F., Paetzold, R., Danel, J., and Sobczyk, L., J. Chem. Soc., Faraday Trans. 2, 1975, vol. 71, p. 1610. https://doi.org/10.1039/F29757101610
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, Jr. J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 09, Pittsburgh PA: Gaussian Inc. 2009.
Funding
The work was financially supported by the Russian Foundation for Basic Research (project no. 20-03-00119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 8, pp. 1120–1131 https://doi.org/10.31857/S0514749221080036.
Supplementary information
Rights and permissions
About this article
Cite this article
Kuznetsova, A.A., Chachkov, D.V., Belogorlova, N.A. et al. Polarity and Conformational Analysis of Tri(1-naphthyl)phosphine, Tri(2-naphthyl)phosphine, and Their Chalcogenides. Russ J Org Chem 57, 1245–1255 (2021). https://doi.org/10.1134/S1070428021080030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021080030