Abstract
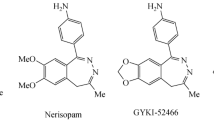
A facile synthetic approach to diazepine and/or benzimidazole derivatives has been developed through the reaction of enamine [N1-(1-phenylethenyl)benzene-1,2-diamine] obtained from o-phenylenediamine and acetophenone. Its reaction with isophthaloyl chloride produced bis(1,5-benzodiazepinyl)benzene derivative via cyclization involving the nucleophilic enamino carbon atom, whereas the cyclocondensation with diethyl succinate occurred at the nucleophilic nitrogen atom to give bis-benzimidazole derivative. Depending on the conditions, the cyclization with carbon disulfide in DMSO afforded tricyclic 4-phenylimidazo[4,5,1-hi]indole-2(1H)-thione, while 4-phenyl-1,3-dihydro-2H-1,5-benzodiazepine-2-thione was formed in the presence of pyridine. Cyclocondensation of o-phenylenediamine with two equivalents of acetophenone in acid medium produced 3-phenyl-1-(1-phenylethenyl)-1,4-dihydroquinoxaline. N-(2-Aminophenyl)thiourea obtained from o-phenylenediamine and ammonium thiocyanate reacted with chloroacetyl chloride, diethyl malonate, carbon disulfide, and sodium nitrite in aqueous HCl to afford benzimidazole, thiadiazolobenzimidazole, and benzotriazole derivatives.




Similar content being viewed by others
REFERENCES
Parlati, F., Ramesh, U.V., Singh, R., Payan, D.G., Lowe, R., and Look, G.C., PCT Int. Appl. no. WO2005037845, 2005. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2005037845
Yoshida, M., Hayakawa, I., Hayashi, N., Agatsuma, T., Oda, Y., Tanzawa, F., Iwasaki, S., Koyama, K., Furukawa, H., Kurakatad, S., and Suganob, Y., Bioorg. Med. Chem. Lett., 2005, vol. 15, p. 3328. https://doi.org/10.1016/j.bmcl.2005.05.077
Bailey, T.R. and Pevear, D.C., PCT Int. Appl. no. WO2004078115, 2004. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2004078115
Alanine, A., PCT Int. Appl. no. WO2001097786, 2001. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2001097786&tab=PCTBIBLIO
Kerwin, S., Hurley, L.H., De Luca, M.R., and Moore, B.M., PCT Int. Appl. no. WO9748694, 1997. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1997048694&_cid=P21-KFFXKU-28472-1
Rathee, P.S., Dhankar, R., Bhardwaj, S., Gupta, M., and Kumar, R., J. Appl. Pharm. Sci., 2011, vol. 1, p. 127. https://www.japsonline.com/admin/php/uploads/73_pdf.pdf
Ali, A., Masumeh, M., Nadia, K., and Somayeh, O., J. Chem. Res., 2015, vol. 39, p. 694. https://doi.org/10.3184/174751915X14473291357176
Shelke, K.F., Badar, A.D., and Devhade, J.B., Chem. Biol. Interface, 2018, vol. 8, p. 22.
Palit, R., Kumar, R., Saraswat, N., Wal, A., and Upadhyaya, P.K., Int. J. Res. Ayurveda Pharm., 2016, vol. 7, pp. 68–73. https://doi.org/10.7897/2277-4343.076243
Shatha, I.A., J. Saudi Chem. Soc., 2017, vol. 21, p. 229. https://doi.org/10.1016/j.jscs.2016.08.001
Cano, N.H., Uranga, J.G., Nardi, M., Procopio, A., Wunderlin, D.A., and Santiago, A.N., Beilstein J. Org Chem., 2016, vol. 12, p. 2410. https://doi.org/10.3762/bjoc.12.235
Alasmary, F.A.S., Snelling, A.M., Zain, M.E., Alafeefy, A.M., Awaad, A.S., and Karodia, N., Molecules, 2015, vol. 20, p. 15206. https://doi.org/10.3390/molecules200815206
Wang, X.-J., Zhang, L., Xu, Y., Krishnamurthy, D., and Senanayake, C.H., Tetrahedron Lett., 2004, vol. 45, p. 7167. https://doi.org/10.1016/j.tetlet.2004.07.042
Divya, B., Ramesh, K., and Poornima, N., Int. J. Pharm. Bio. Sci., 2012, vol. 2, p.143.
Wan, Z.-K., Ousman, E.F., Papaioannou, N., and Saiah, E., Tetrahedron Lett., 2011, vol. 52, p. 4149. https://doi.org/10.1016/j.tetlet.2011.05.146
Junke, W., Peng, F., Jiang, J.-L., Lu, Z.-J., Wang, L.-Y., Bai, J., and Pan, Y., Tetrahedron, Lett., 2008, vol. 49, p. 467. https://doi.org/10.1016/j.tetlet.2007.11.100
Padmavathy, K., Gopalpur, N., and Geetha, K.V., Tetrahedron Lett., 2011, vol. 52, p. 544. https://doi.org/10.1016/j.tetlet.2010.11.116
Nardi, D., Tajana, A., and Rossi, S., J. Heterocycl. Chem., 1973, vol. 10, p. 815. https://doi.org/10.1002/jhet.5570100524
Beckurts, H., Frerichs, G., and Hupka, H., Arch. Pharm., 1903, vol. 241, p. 161. https://doi.org/10.1002/ardp.19032410302
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Shehta, W., Assy, M.G., Ismail, N.A. et al. o-Phenylenediamine as a Source of Fused Azole, Azine, and Diazepine Derivatives. Russ J Org Chem 57, 1152–1157 (2021). https://doi.org/10.1134/S1070428021070150
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021070150