Abstract
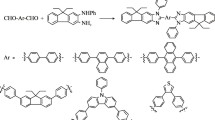
A series of novel coronene-based fluorescent materials have been synthesized through well-defined protocol from 1,2-dimethoxybenzene, with the key steps including Suzuki–Miyaura coupling and oxidative cyclodehydrogenation with ferric chloride. Fluorescence spectra investigations proved that they have emissions in the range λ 460-470 nm with excitation wavelength at λ 373 nm. It is found that different chromophores from substituents can significantly enhance the fluorescence intensities, which paves a new way of application of these materials in various fields.



Similar content being viewed by others
REFERENCES
Wei, J.F., Han, B., Guo, Q., Shi, S.Y., Wang, W.L., and Wei, N., Angew. Chem., Ind. Ed., 2010, vol. 49, p. 8209. https://doi.org/10.1002/anie.201002369
Johns, J.E., Muller, E.A., Fréchet, J.M.J., and Harris, C.B., J. Am. Chem. Soc., 2010, vol. 132, p. 15720. https://doi.org/10.1021/ja1066866
Wang, J.L., Zhou, Y., Li, Y., and Pei, J., J. Org. Chem., 2009, vol. 74, p. 7449. https://doi.org/10.1021/jo901539a
Ma, B., Woo, C.H., Miyamoto, Y., and Fréchet, J.M.J., Chem. Mater., 2009, vol. 21, p. 1413. https://doi.org/10.1021/cm900005g
Li, Z.T., Zhi, L.J., Lucas, N.T., and Wang, Z.H., Tetrahedron, 2009, vol. 65, p. 3417. https://doi.org/10.1016/j.tet.2009.02.042
Wu, J.S., Grimsdale, A.C., and Müllen, K., J. Mater. Chem., 2005, vol. 15, p. 41. https://doi.org/10.1039/B413115K
Yamamoto, Y., Fukushima, T., Suna, Y., Ishii, N., Saeki, A., Seki, S., Tagawa, S., Tanihuchi, M., Kawai, T., and Aida, T., Science, 2006, vol. 314, p. 1761. https://doi.org/10.1126/science.1134441
Schmidt-Mende, L., Fechtenkötter, A., Müllen, K., Moons, E., Friend, R.H., MacKenzie, J.D., Science, 2001, vol. 293, p. 1119. https://doi.org/10.1126/science.293.5532.1119
Wong, W.W.H., Jones, D.J., Yan, C., Watkins, S.E., King, S., Haque, S.A., Wen, X.M., Ghiggino, K.P., and Holmes, A.B., Org. Lett., 2009, vol. 11, p. 975. https://doi.org/10.1021/ol8029164
Zhang, R.F., Zheng, H.P., and Shen, J.C., Synth. Met., 1999, vol. 105, p. 49. https://doi.org/10.1016/S0379-6779(99)00056-9
Li, Z.T., Lucas, N.T., Wang, Z.H., and Zhu, A.B., J. Org. Chem., 2007, vol. 72, p. 3917. https://doi.org/10.1021/jo0701029
Li, Z.T., Hu, Z.P., Chen, X., Zhang, Y.P., and Zhang, J.M., Chem. Lett., 2012, vol. 41, p. 1588. https://doi.org/10.1246/cl.2012.1588
Wang, X.D. and Wolfbeis, O.S., Chem. Soc. Rev., 2014, vol. 43, p. 3666. https://doi.org/10.1039/C4CS00039K
Chee, G.J., Nomura, Y., Ikebukuro, K., and Karube, I., Biosens. Bioelectron., 2000, vol. 15, p. 371. https://doi.org/10.1016/S0956-5663(00)00093-2
Fujiwara, Y. and Amao, Y., Sens. Actuators, B, 2003, vol. 89, p. 58. https://doi.org/10.1016/S0925-4005(02)00428-8
Papkovsky, D.B. and Dmitriev, R.I., Chem. Soc. Rev., 2013, vol. 42, p. 8700. https://doi.org/10.1039/C3CS60131E
Boden, N., Bushby, R.J., Cammidge, A.N., Duckworth, S., and Headdock, G., J. Mater. Chem., 1997, vol. 7, p. 601. https://doi.org/10.1039/A606447G
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declared the absence of conflict of interest.
Supplementary material
Rights and permissions
About this article
Cite this article
Tang, G., An, S., Zhong, Y. et al. Novel Large π-Conjugated Coronene-Based Molecules: Diaryl-Substituted 1,2,7,8,13,14-Hexamethoxytribenzo[a,g,m]coronenes. Russ J Org Chem 56, 1779–1783 (2020). https://doi.org/10.1134/S1070428020100188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020100188