Abstract
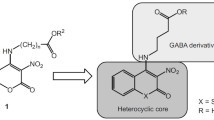
A series of novel 2-(4-nitrophenyl)-3-(R-benzothiazol-2-yl)quinazolin-4(3H)-ones 4a–4g (R = Alk, AlkO, Hal, NO2) were synthesized from the corresponding substituted 2-aminobenzothiazoles and 2-(4-nitrophenyl)-4H-3,1-benzoxazin-4-one via a nucleophilic addition reaction. The structures of the novel compounds were assigned on the basis of the elemental analyses and FTIR, 1H NMR, and mass spectra. The synthesized compounds were tested for anticonvulsant, antimicrobial, and antioxidant activities. All the compounds increased the seizure latency compared to control. Compound 4b (R = 6-NO2) exhibited significant anticonvulsant activity, comparable to that of the standard drug Phenytoin. Antimicrobial activity testing revealed moderate to good activity in all the test compounds, and 4a (R = H) and 4b compared in activity with the standard drug Chloramphenicol. The antioxidant activity of compounds 4a, 4d (R = 5-Br), and 4f (R = 4,6-Me2) (IC50 39.30, 15.55, and 42.95 µg/mL, respectively) was found to be higher compared to the standard drug ascorbic acid (IC50 48.30 µg/mL).

Similar content being viewed by others
REFERENCES
Kusuma, P.K. and Vedula, G., Biosci. Biotech. Res. Asia, 2016, vol. 13, p. 1121. https://doi.org/10.13005/bbra/2141
Mishra, A.D., Nepal J. Sci. Technol., 2011, vol. 12, p. 133. https://doi.org/10.3126/njst.v12i0.6491
Abuelizz, H.A., El Dib, R., Marzouk, M., Anouar, El-H., Maklad, Y.A., Attia, H.N., and Al-Salahi, R., Molecules, 2017, vol. 22, p. 1. https://doi.org/10.3390/molecules22071094
Hosseinzadeh, L., Aliabadi, A., Rahnama, M., Sadeghi, H.M.M., and Khajouei, M.R., Res. Pharm. Sci., 2017, vol. 12, p. 290. https://doi.org/10.4103/1735-5362.212046
Saeedi, M., Mohammadi-Khanaposhtani, M., Pourrabia, P., Razzaghi, N., Ghadimi, R., Imanparast, S., Faramarzi, M.A., Bandarian, F., Esfahani, E.N., Safavi, M., Rastegar, H., Larijani, B., Mahdavi, M., and Akbarzadeh, T., Bioorg. Chem., 2019, vol. 83, p. 161. https://doi.org/10.1016/j.bioorg.2018.10.023
Osarumwense, P.O., Edema, M.O., and Usifoh, O., IOSR-JAC, 2018, vol. 11, p. 12. https://doi.org/10.9790/5736-1104011215
Hosseinzadeh, L., Aliabadi, A., Kalantari, M., Mostafavi, A., and Khajouei, M.R., Res. Pharm. Sci., 2016, vol. 11, p. 210.
Karumanchi, A.D., Sarangapani, M., and Sriram, Int. J. Drug Dev. Res., 2014, vol. 6, p. 259.
Ibrahim, M.K., El-Adl, K., and Al-Karmalawy, A.A., Bull. Fac. Pharm. Cairo Univ., 2015, vol. 53, p. 101–116. https://doi.org/10.1016/j.bfopcu.2015.05.001
Paneersalvam, P., Raj, T., Ishar, M.P.S., Singh, B., Sharma, V., and Rather, B.A., Indian. J. Pharm. Sci., 2010, vol. 72, p. 375. https://doi.org/10.4103/0250-474X.70488
Zayed, M.F. and Hassan, M.H., Saudi Pharm. J., 2014, vol. 22, p. 157. https://doi.org/10.1016/j.jsps.2013.03.004
Pawar, S., Pisal, P., Deodhar, M., Kale, A., and Nigade, G., Int. J. Pharm. Pharm. Sci., 2018, vol. 10, p. 57. https://doi.org/10.22159/ijpps.2018v10i10.28480
Prabhu, P.P., Panneerselvam, T., Shastry, C.S., Sivakumar, A., and Pande, S.S., J. Saudi Chem. Soc., 2015, vol. 19, p. 181. https://doi.org/10.1016/j.jscs.2012.02.001
Ajeet and Kumar, A., Am. J. Pharm. Sci., 2013, vol. 1, p. 116. https://doi.org/10.12691/ajps-1-6-2
Gupta, A., Asian. J. Pharm. Clin. Res., 2018, vol. 11, p. 416. https://doi.org/10.22159/ajpcr.2018.v11i9.28095
Shakya, A.K., Kumar, A., and Siddiqui, H.H., Indian J. Heterocycl. Chem., 2016, vol. 25, p. 243.
Dhamak, K.B., Gaware, V.M., and Somwanshi, S.B., Int. J. Pharm. Pharm. Res., 2015, vol. 3, p. 112.
Gollapalli, N., Karumudi, B.S., Kota, C., Gudipati, M., Suresh, P.V., and Ramarao, N., Indo Am. J. Pharm. Res., 2015, vol. 5, p. 1288.
Devmurari, V.P., Pandey, S., Goyani, M.B., Nandanwar, R.R., Jivani, N.P., and Perumal, P., Int. J. ChemTech. Res., 2010, vol. 2, p. 681.
Kabra, U., Chopde, C., and Wadodkar, S., J. Heterocycl. Chem., 2011, vol. 48, p. 1351. https://doi.org/10.1002/jhet.754
Ugale, V.G., Wadodkar, S.G., and Chopde, C.T., Asian J. Res. Chem., 2011, vol. 4, p. 1717.
Ganguli, S., Panigrahi, M.K., Singh, P., and Shukla, P.K., Int. J. Pharm. Pharm. Sci., 2012, vol. 4, p. 434.
Laddha, S.S., Wadodkar, S.G., and Meghal, S.K., Arkivoc, 2006, vol. 11, p. 1. https://doi.org/10.3998/ark.5550190.0007.b01
Vogel, H.G., Drug Discovery and Evaluation Pharmacological Assays, New York: Springer, 2002.
Upadhaye, G.J. and Asnani, A.J., IJPSR, 2017, vol. 8, p. 1214. https://doi.org/10.13040/IJPSR.0975-8232.8(3).1314-18
Kumar, C.T.K., Keshavayya, J., Ravi, B.N., and Patil, S.R., Int. J. Innov. Res. Dev., 2016, vol. 5, p. 13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors is no conflict of interest.
Rights and permissions
About this article
Cite this article
Giradkar, V.N., Kabra, U.D., Diwakar, R.S. et al. Synthesis, Characterization, and Biological Evaluation of 2-(p-Nitrophenyl)quinazolin-4(3H)-one Derivatives. Russ J Org Chem 56, 1455–1461 (2020). https://doi.org/10.1134/S1070428020080175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020080175