Abstract
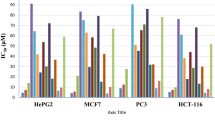
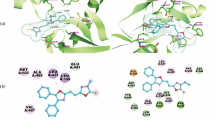
The 1,3-dipolar cycloaddition of nitrile imines to 11-aryl-4-(arylmethylidene)-1,2,3,4,11,11a-hexahydrodibenzo[b,e][1,4]thiazepines possessing exocyclic C=C and endocyclic C=N bonds as dipolarophilic sites showed site selectivity, depending on the type of C-substituent in the nitrile imine. 1,3-Dipolar cycloaddition of C-aryl nitrile imines occurred selectively to the exocyclic C=C bond, whereas the endocyclic C=N bond was involved in the cycloaddition with C-ethoxycarbonyl nitrile imines. A combination of total energy and molecular orbital plots for the highest occupied and lowest unoccupied molecular orbitals was used to verify the proposed reaction mechanisms and stereoselectivity. Some of the isolated products exhibited moderate to good antitumor activity. The results of POM analysis of the relative cytotoxicity of these new derivatives in comparison to Doxorubicin are also reported.













Similar content being viewed by others
REFERENCES
Shawali, A.S., Curr. Org. Chem., 2014, vol. 18, p. 598. https://doi.org/10.2174/1385272819666140201002900
Papdopoulos, S. and Stephanidou, J.S., J. Heterocycl. Chem., 1987, vol. 24, p. 309. https://doi.org/10.1002/jhet.5570240203
Baba, N. and Soufiaoui, M., Tetrahedron Lett., 1990, vol. 31, p. 1709. https://doi.org/10.1016/S0040-4039(00)88860-7
Liu, B., Li, X-F., Liu, H-C., and Yu, X.-Y., Tetrahedron Lett., 2013, vol. 54, p. 6952. https://doi.org/10.1016/j.tetlet.2013.10.062
Abdel Hafez, N.A., Hassaneen, H.M.E., Farghaly, T.A., Riyadh, S., and Alzahaby, H., Mini-Rev. Med. Chem., 2018, vol. 18, p. 631. https://doi.org/10.2174/1389557517666170912170027
Shneine, J.K. and Alaraji, Y.H., Int. J. Sci. Res., 2016, vol. 5, no. 3, p. 1411. https://www.ijsr.net/archive/v5i3/NOV161902.pdf
Capozzi, G., Chimirri, A., Grasso, S., Romeo, G., and Zappis, G., Heterocycles, 1985, vol. 23, p. 2051. https://doi.org/10.3987/R-1985-08-2051
Farghaly, T.A., Gomha, S.M., Sayed, A.R., and Khedr, M.A., Curr. Org. Synth., 2016, vol. 13, p. 445. https://doi.org/10.2174/1570179412666150817220018
Shawali, A.S., Farghaly, T.A., and Nawar, T.M.S., J. Heterocycl. Chem., 2016, vol. 53, p. 909. https://doi.org/10.1002/jhet.2151
Kassem, A.F., Alshehrei, F., Abbas, E.M.H., and Farghaly, T.A., Mini-Rev. Med. Chem., 2020, vol. 20, p. 418. https://doi.org/10.2174/1389557519666190603091101
Farghaly, T.A., Abdallah, M.A., and Muhammad, Z.A., Curr. Org. Synth., 2016, vol. 13, p. 291. https://doi.org/10.2174/1570179412666150706183544
Farghaly, T.A., Abdallah, M.A., Masaret, G.S.,and Muhammad, Z.A., Eur. J. Med. Chem., 2015, vol. 97, p. 320. https://doi.org/10.1016/j.ejmech.2015.05.009
Farghaly, T.A. and Mahmoud, H.K., J. Heterocycl. Chem., 2015, vol. 52, p. 86. https://doi.org/10.1002/jhet.1985
Abdel Hafez, N.A., Farghaly, T.A., Al-Omar, M.A., and Abdalla, M.M., Eur. J. Med. Chem., 2010, vol. 45, p. 4838. https://doi.org/10.1016/j.ejmech.2010.07.053
Shawali, A.S. and Farghaly, T.A., Arkivoc, 2008, vol. 2008, part (i), p. 18. www.arkat-usa.org/get-file/23038/
Farghaly, T.A. and Mahmoud, H.K., Arch. Pharm. (Weinheim, Ger.), 2013, vol. 346, p. 392. https://doi.org/10.1002/ardp.201200486
Dawood, K.M., Tetrahedron, 2005, vol. 61, p. 5229. https://doi.org/10.1016/j.tet.2005.03.083
Riyadh, S.M. and Farghaly, T.A., Tetrahedron, 2012, vol. 68, p. 9056. https://doi.org/10.1016/j.tet.2012.08.064
Hakkou, Z., Maciuk, A., Leblais, V., Bouanani, N.E., Mekhfi, H., Bnouham, M., Aziz, M., Ziyyat, A., Rauf, A., Ben Hadda, T., Shaheen, U., Patel, S., Fischmeiste, R., and Legssyer A., Biomed. Pharmacother., 2017, vol. 93, p. 62. https://doi.org/10.1016/j.biopha.2017.06.015
Rauf, A., Uysal, S., Ben Hadda, T., Siddiqui, B.S., Khan, H., Atif Khan, M., Ijaz, M.I., Mubarak, M.S., Bawazeer, S., Abu-Izneid, T., Khan, A., and Farooq, U., Marmara Pharm. J., 2017, vol. 21, p. 261. https://doi.org/10.12991/marupj.300335
Genc, M., Karagoz Genc, Z., Tekin, S., Sandal, S., Sirajuddin, M., and Ben Hadda, T., Acta Chim. Slov., 2016, vol. 63, p. 726. https://doi.org/10.17344/acsi.2016.2428
Mabkhot, Y.N., Arfan, M., Zgou, H., Genc, Z.K., Genc, M., Rauf, A., Bawazeer, S., and Ben Hadda, T. Res. Chem. Intermed., 2016, vol. 42, p. 8055. https://doi.org/10.1007/s11164-016-2578-8
Rauf, A., Uddin, G., Siddiqui, B.S., Khan, H., Mujeeb-ur-Rehman., Warad, I., Ben Hadda, T., Patel, S., Khan, A., and Farooq, U., Curr. Bioactive Compd., 2015, vol. 11, p. 231. https://doi.org/10.2174/1573407211666151012191902
Mabkhot, Y.N., Alatibi, A., El-Sayed, N., Kheder, N., Wadood, A., Rauf, A., Bawazeer, S., Al-Showiman, S., and Ben Hadda, T., Molecules, 2016, vol. 21, p. 222. https://doi.org/10.3390/molecules21020222
Tatar, E., Şenkardeş, S., Sellitepe, H.E., Güniz Küçükgüzel, Ş., Karaoğlu, S.A., Bozdeveci, A., De Clercq, E., Pannecouque, C., Ben Hadda, T., and Küçükgüzel, İ., Turk. J. Chem., 2016, vol. 40, p. 510. https://doi.org/10.3906/kim-1509-21
Al-Maqtari, H.M., Jamalis, J., Ben Hadda, T., Sankaranarayanan, M., Chander, S., Ahmad, N.A., Sirat, H.M., Althagafi, I.I., and Mabkhot, Y.N., Res. Chem. Intermed., 2017, vol. 43, p. 1893. https://doi.org/10.1007/s11164-016-2737-y
Sajid, Z., Ahmad, M., Aslam, S., Ashfaq, U.A., Zahoor, A.F., Saddique, F.A., Parvez, M., Hameed, A., Sultan, S., Zgou, H., and Ben Hadda, T., Pharm. Chem. J., 2016, vol. 50, p. 172. https://doi.org/10.1007/s11094-016-1417-y
Amirkhanov, V., Rauf, A., Ben Hadda, T., Ovchynnikov, V., Trush, V., Saleem, M., Raza, M., Rehman, T., Zgou, H., Shaheen, U., and Farghaly, T.A., Mini-Rev. Med. Chem., 2019, vol. 19, p. 1015. https://doi.org/10.2174/1389557519666190222172757
Pervez, H., Ahmad, M., Ben Hadda, T., Toupet, L., and Naseer, M.M., J. Mol. Struct., 2015, vol. 1098, p. 124. https://doi.org/10.1016/j.molstruc.2015.06.013
Abdelhady, M.I.S., Kamal, A.M., Rauf, A., Mubarak, M.S., and Ben Hadda, T., Nat. Prod. Res., 2016, vol. 30, p. 1131. https://doi.org/10.1080/14786419.2015.1045508
Header, E., El-Sawy, N., El-Boshy, M., Basalamah, M., Mubarak, M.S., and Ben Hadda, T., J. Bioanal. Biomed., 2016, vol. 15, p. 18. https://doi.org/10.4172/1948-593X.1000118
Ben Hadda, T., Genc, Z.K., Masand, V.H., Nebbache, N., Warad, I., Jodeh, S., Genc, M., Mabkhot, Y.N., Barakat, A., and Salgado-Zamora, H., Acta Chim. Slov., 2015, vol. 62, p. 679. https://doi.org/10.17344/acsi.2015.1357
Hatzade, K., Sheikh, J., Taile, V., Ghatole, A., Ingle, V., Genc, M., Lahsasni, S., and Ben Hadda, T., Med. Chem. Res., 2015, vol. 24, p. 2679. https://doi.org/10.1007/s00044-015-1326-8
Lipinski, C.A., Lombardo, F., Dominy, B.W., and Feeney, P.J., Adv. Drug Delivery Rev., 2001, vol. 46, p. 3. https://doi.org/10.1016/S0169-409X(00)00129-0
Kaugars, G., Gemrich, E.G., and Rizzo, V.L., J. Agric. Food Chem., 1973, vol. 21, p. 647. https://doi.org/10.1021/jf60188a051
Hassaneen, H.M., Mousa, H.A.H., Abed, N.M., and Shawali, A.S., Heterocycles, 1988, vol. 27, p. 695. https://doi.org/10.3987/COM-87-4381
Shawali, A.S. and Albar, H.A., Can. J. Chem., 1986, vol. 64, p. 871. https://doi.org/10.1139/v86-144
Becke, A.D., Phys. Rev. A, 1988, vol. 38, p. 3098. https://doi.org/10.1103/PhysRevA.38.3098
Becke, A.D., J. Chem. Phys., 1993, vol. 98, p. 5648. https://doi.org/10.1063/1.464913
Arjunan, V., Suja Ravi Isaac, A., Rani, T., Mythili, C.V., and Mohan, S., Spectrochim. Acta, Part A, 2011, vol. 78, p. 1625. https://doi.org/10.1016/j.saa.2011.02.018
Lee, C., Yang, W., and Parr, R.G., Phys. Rev. B, 1988, vol. 37, p. 785. https://doi.org/10.1103/PhysRevB.37.785
McLean, A.D. and Chandler, G.S., J. Chem. Phys., 1980, vol. 72, p. 5639. https://doi.org/10.1063/1.438980
Krishnan, R., Binkley, J.S., Seeger, R., and Pople, J.A., J. Chem. Phys., 1980, vol. 72, p. 650. https://doi.org/10.1063/1.438955
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., MontgomeryJr, J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., and Fox, D.J., Gaussian 09W, Wallingford CT: Gaussian, 2010.
Dennington, R., Keith, T., and Millam, J., Gauss View, version 5, Shawnee Mission KS: Semichem, 2009.
Vijayan, P., Raghu, C., Ashok, G., Dhanaraj, S.A., and Suresh, B., Indian J. Med. Res., 2004, vol. 120, p. 24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Farghaly, T.A., Abbas, I.M., Hassan, W.M.I. et al. Structure Determination and Quantum Chemical Analysis of 1,3-Dipolar Cycloaddition of Nitrile Imines and New Dipolarophiles and POM Analyses of the Products as Potential Breast Cancer Inhibitors. Russ J Org Chem 56, 1258–1271 (2020). https://doi.org/10.1134/S1070428020070210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020070210