Abstract
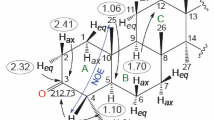
An effective reagent (performic acid) was proposed for the Bayer–Villiger oxidation of glycyrrhetic acid methyl ester to the corresponding oxepan-2-one. The low-temperature (–70°C) reduction of the latter with excess (5 equiv) diisobutylaluminium hydride in CH2Cl2 resulted in the isolation of 3,4-seco-18β-oleane-4(23),9(11),12-triene-3,30-diol as the main product, while the expected (R)-3,4-epoxy-3-isobutoxy-3,4-seco-18β-oleane-11,12-dien-30-ol was the minor product.



Similar content being viewed by others
REFERENCES
Tosltikov, G.A., Baltina, L.A., and Grankina, V.P., Solodka: bioraznoobrazie, khimiya, primenenie v meditsine (Licorice: Biodiversity, Chemistry, Medical Applications), Novosibirsk: Geo, 2007.
Beseda, I., Czollner, L., Shah, P.S., Khunt, R., Gaware, R., Kosma, P., Stanetty, Ch., Carmen del Ruiz-Ruiz, M., Amer, H., Mereiter, K., Da Cunha, T., Odermatt, A., Classen-Houben, D., and Jordis, U., Bioorg. Med. Chem., 2010, vol. 18, p. 433. https://doi.org/10.1016/j.bmc.2009.10.036
Maitraie, D., Hung, C.-F., Tu, H.-Y., Liou, Y.-T., Wei, B.-L., Yang, S.-C., Wang, J.-P., and Lin, Ch.-N., Bioorg. Med. Chem., 2009, vol. 17, p. 2785. https://doi.org/10.1016/j.bmc.2009.02.025
Ishmuratov, G.Yu., Yakovleva, M.P., Vydrina, V.A., Khasanova, E.F., Muslukhov, R.R., Ishmuratova, N.M., and Tolstikov, G.A., Khim. Rast. Syrya, 2007, p. 23.
Ishmuratov, G.Yu., Vydrina, V.A., Yakovleva, M.P., Valeeva, E.F., Muslukhov, R.R., and Tolstikov, G.A., Russ. J. Org. Chem., 2011, vol. 47, p. 472. https://doi.org/10.1134/S1070428011030304
Ishmuratov, G.Yu., Vydrina, V.A., Galkina, Yu.A., Yakovleva, M.P., Muslukhov, R.R., and Tolstikov, G.A., Russ. J. Org. Chem., 2014, vol. 50, p. 1704. https://doi.org/10.1134/S1070428014110311
Vydrina, V.A., Kravchenko, A.A., Denisova, K.S., Yakovleva, M.P., and Ishmuratov, G.Yu., Chem. Nat.Compd., 2016, vol. 52, p. 959. https://doi.org/10.1007/s10600-016-1833-y
Baltina, L.A., Flekhter, O.B., Putieva, Zh.M., Kondratenko, R.M., Krasnova, L.V., and Tolstikov, G.A., Pharm. Chem. J., 1996, vol. 30, p. 263. https://doi.org/10.1007/BF02333971
Gordon, A. and Ford, R., Chemist's Companion, New York: Wiley, 1972.
ACKNOWLEDGMENTS
The work was performed using the equipment of the Khimiya Center for Collective Use, Ufa Institute of Chemistry, Ufa Research Center, Russian Academy of Sciences.
Funding
The work was financially supported by the “Fundamentals of Chemistry” Program of the Russian Academy of Sciences, topic no. 8 “Chemo-, Regio-, and Stereoselective Transformations of Terpenoids, Steroids, and Lipids in the Directed Synthesis of Low-Molecular-Weight Bioregulators” (State Registration no. AAAA-A17-117011910023-2, 2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Vydrina, V.A., Kravchenko, A.A., Sataraev, D.A. et al. Synthesis and Properties of Methyl 3,4-Epoxy-3,11-dioxo-3,4seco-18β-olean-12-ene-30-carboxylate in a New Reaction of Organoaluminium Compounds. Russ J Org Chem 56, 251–254 (2020). https://doi.org/10.1134/S1070428020020116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020020116