Abstract
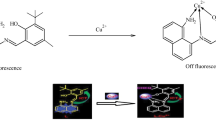
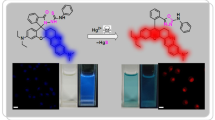
A novel multichromophoric hybrid compound, 2-[1-(4-tert-butylphenyl)-4,5-diphenyl-1H-imidazol-2-yl]-6-(pyren-1-yl)quinoline (TDIPQ) has been synthesized as an ON-OFF fluorescent chemosensor for copper(II) ions. Colorless TDIPQ in acetonitrile—water (2:1, v/v) selectively turns yellow along with fluorescence quenching upon addition of copper(II) ions. The fluorescence quenching is directly proportional to the concentration of copper(II) ions. The interaction between TDIPQ and copper(II) was investigated with the aid of UV-Vis, fluorescence, 1H NMR, and MALDI mass spectral techniques. The stoichiometry of the TDIPQ—Cu complex was determined to be 2:1 by Job’s Plot. Under similar experimental conditions, other competitive metal ions had negligible or no interference in the detection ability of TDIPQ. The detection and quantification limits of TDIPQ were estimated at 2 × 10−6 M and 6.2 × 10−6 M. respectively. This method showed an excellent precision of 0.98 ± 0.011 and recovery characteristic of 99.09±1.4%. It is applicable for the quantification of copper(II) in various samples such as drinking water, lab waste water, and soil. A mixture of TDIPQ with the BZA-Co-BZMA polymer can be cast as a film on a glass slide to be used as a sensor device to indicate the presence of copper. Polymer-coated TDIPQ chemosensing property was analyzed by SEM imaging.
Similar content being viewed by others
References
Chowdhury, S., Rooj, B., Dutta, A., and Mandal, U., J. Fluoresc., 2018, vol. 28, no. 4, p. 999. https://doi.org/10.1007/s10895-018-2263-y
Maiti, D., Lucas, H.R., Sarjeant, A.A.N., and Karlin, K.D., J. Am. Chem. Soc., 2007, vol. 129, no. 22, p. 6998. https://doi.org/10.1021/ja071704c
Hahn, S.H., Tanner, M.S., Danke, D.M., and Gahl, W.A., Biochem. Mol. Med., 1995, vol. 54, no. 2, p. 142. https://doi.org/10.1006/bmme.1995.1021
Zietz, B.P., de Vergara, J.D., and Dunkelberg, H., Environ. Res., 2003, vol. 92, no. 2, p. 129. https://doi.org/10.1016/S0013-9351(03)00037-9
Deraeve, C., Boldron, C., Maraval, A., Mazarguil, H., Gornitzka, H., Vendier, L., Pitie, M., and Meunier, B., Chem. Eur. J., 2008, vol. 14, no. 2, p. 682. https://doi.org/10.1002/chem.200701024
Lee, J.C., Gray, H.B., and Winkler, J.R., J. Am. Chem. Soc., 2008, vol. 130, no. 22, p. 6898. https://doi.org/10.1021/ja711415b
World Health Organization. Guidelines for Drinking-Water Quality, Geneva, 2008, 3rd ed.
Paulose, P.I., Jose, G., Thomas, V., Jose, G., Unnikrishnan, N.V., and Warrier, M.K.R., Bull. Mater. Sci., 2002, vol. 25, no. 1, p. 69. https://doi.org/10.1007/BF02704598
Saravu, K., Jose, J., Bhat, M.N., Jimmy, B., and Shastry, B.A., Indian J. Crit. Care Med., 2007, vol. 11, no. 2, p. 74. https://doi.org/10.4103/0972-5229.33389
Gonzales, A.P.S., Firmino, M.A., Nomura, C.S., Rocha, F.R.P., Oliveira, P.V., and Gaubeur, I., Anal. Chim. Acta, 2009, vol. 636, no. 2, p. 198. https://doi.org/10.1016/j.aca.2009.01.047
Becker, J.S., Matusch, A., Depboylu, C., Dobrowolska, J., and Zoriy, M.V., Anal. Chem., 2007, vol. 79, no. 16, p. 6074. https://doi.org/10.1021/ac0700528
Liu, Y., Liang, P., and Guo, L., Talanta, 2005, vol. 68, no. 1, p. 25. https://doi.org/10.1016/j.talanta.2005.04.035
Ensafi, A.A., Khayamian, T., Benvidi, A., and Mirmomtaz, E., Anal. Chim. Acta, 2006, vol. 561, nos. 1–2, p. 225. https://doi.org/10.1016/j.aca.2006.01.015
Cheng, Y., Feng, Q., Yin, M., Wang, C., and Zhou, Y., Tetrahedron Lett., 2016, vol. 57, no. 34, p. 3814. https://doi.org/10.1016/j.tetlet.2016.07.013
Kaewtong, C., Pulpoka, B., and Tuntulani, T., Dyes Pigm., 2015, vol. 123, p. 204. https://doi.org/10.1016/j.dyepig.2015.08.001
Li, N., Zong, L., Li, Q., and Li, Z., Sens. Actuators, B, 2015, vol. 207, p. 827. https://doi.org/10.1016/j.snb.2014.10.118
Mohammadi, A. and Yaghoubi, S., Sens. Actuators, B, 2017, vol. 241, p. 1069. https://doi.org/10.1016/j.snb.2016.10.034
Singh, A. and Ramanathan, G., J. Lumin., 2017, vol. 182, p. 220. https://doi.org/10.1016/j.jlumin.2016.10.024
Tang, L., Cai, M., Huang, Z., Zhong, K., Hou, S., Bian, Y., and Nandhakumar, R., Sens. Actuators, B, 2013, vol. 185, p. 188. https://doi.org/10.1016/j.snb.2013.04.109
Eseola, A.O., Li, W., Adeyemi, O.G., Obi-Egbedi, N.O., and Woods, J.A., Polyhedron, 2010, vol. 29, no. 8, p. 1891. https://doi.org/10.1016/j.poly.2010.02.039
Feng, K., Hsu, F.L., Van DerVeer, D., Bota, K., and Bu, X.R., J. Photochem. Photobiol., A, 2004, vol. 165, nos. 1–3, p. 223. https://doi.org/10.1016/j.jphotochem.2004.03.021
Chereddy, N.R., Saranraj, K., Barui, A.K., Patra, C.R., Rao, V.J., and Thennarasu, S., RSC Adv., 2014, vol. 4, no. 46, p. 24324. https://doi.org/10.1039/C4RA02797C
Chereddy, N.R., Raju, M.N., Nagaraju, P., Krishnaswamy, V.R., Korrapati, P.S., Bangal, P.R., and Rao, V.J., Analyst, 2014, vol. 139, no. 24, p. 6352. https://doi.org/10.1039/C4AN01528B
Chereddy, N.R., Nagaraju, P., Raju, M.N., Saranraj, K., Thennarasu, S., and Rao, V.J., Dyes Pigm., 2015, vol. 112, p. 201. https://doi.org/10.1016/j.dyepig.2014.07.004
Chereddy, N.R., Nagaraju, P., Raju, M.N., Krishnaswamy, V.R., Korrapati, P.S., Bangal, P.R., and Rao, V.J., Biosens. Bioelectron., 2015, vol. 68, p. 749. https://doi.org/10.1016/j.bios.2015.01.074
Harsha, K.G., Appalanaidu, E., Chereddy, N.R., Baggi, T.R., and Rao, V.J., Sens. Actuators, B, 2018, vol. 256, p. 528. https://doi.org/10.1016/j.snb.2017.10.120
Calvin, M. and Bailes, R.H., J. Am. Chem. Soc., 1946, vol. 68, no. 6, p. 949. https://doi.org/10.1021/ja01210a012
Adamson, A.W., J. Am. Chem. Soc., 1954, vol. 76, no. 6, p. 1578. https://doi.org/10.1021/ja01635a030
Vallet, V., Wahlgren, U., and Grenthe, I., J. Am. Chem. Soc., 2003, vol. 125, no. 48, p. 14941. https://doi.org/10.1021/ja036646j
Vyas, P.V., Bhatt, A.K., Ramachandraiah, G., and Bedekar, A.V., Tetrahedron Lett., 2003, vol. 44, no. 21, p. 4085. https://doi.org/10.1016/S0040-4039(03)00834-7
Wu, C., Wang, J., Shen, J., Zhang, C., Wu, Z., and Zhou, H., Tetrahedron, 2017, vol. 73, no. 38, p. 5715. https://doi.org/10.1016/j.tet.2017.08.010
Chereddy, N.R., Thennarasu, S., and Mandal, A.B., Sens. Actuators, B, 2012, vol. 171, p. 294. https://doi.org/10.1016/j.snb.2012.03.077
Goh, H., Ko, Y.G., Nam, T.K., Singh, A., Singh, N., and Jang, D.O., Tetrahedron Lett., 2016, vol. 57, no. 39, p. 4435. https://doi.org/10.1016/j.tetlet.2016.08.074
More, P.A. and Shankarling, G.S., Sens. Actuators, B, 2017, vol. 241, p. 552. https://doi.org/10.1016/j.snb.2016.10.121
Wang, H., Shi, D.L., Li, J., Tang, H.Y., Li, J., and Guo, Y., Sens. Actuators, B, 2018, vol. 256, p. 600. https://doi.org/10.1016/j.snb.2017.10.124
Parsaee, Z., Haratipour, P., Lariche, M.J., and Vojood, A., Ultrason. Sonochem., 2018, vol. 41, p. 337. https://doi.org/10.1016/j.ultsonch.2017.09.054
Chereddy, N.R., Raju, M.N., Reddy, B.M., Krishnaswamy, V.R., Korrapati, P.S., Reddy, B.J.M., and Rao, V.J., Sens. Actuators, B, 2016, vol. 237, p. 605. https://doi.org/10.1016/j.snb.2016.06.131
Job, P., Ann. Chim. Appl., 1928, vol. 9, p. 113.
Banerjee, A., Karak, D., Sahana, A., Guha, S., Lohar, S., and Das, D., J. Hazard. Mater., 2011, vol. 186, no. 1, p. 738. https://doi.org/10.1016/j.jhazmat.2010.11.060
Acknowledgments
K.G. Harsha and E. Appalanaidu thank UGC-New Delhi and CSIR-Delhi, respectively, for the financial assistance. B.A. Rao thank CSIR-Delhi for fellowship as CSIR-RA. V.J. Rao thanks CSIR-Delhi for Emeritus Scientist honor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harsha, K.G., Appalanaidu, E., Rao, B.A. et al. ON–OFF Fluorescent Imidazole Derivative for Sensitive and Selective Detection of Copper(II) Ions. Russ J Org Chem 56, 158–168 (2020). https://doi.org/10.1134/S1070428020010248
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020010248