Abstract
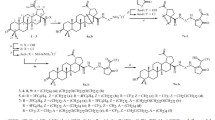
The reaction of 30-bromo-3β,28-diacylbetulin with sodium azide afforded 30-azido-3β,28-diacyloxylup-20(29)-enes. The products were subjected to a CuI/TMEDA-catalyzed click reaction with ethynylferrocene to obtain the corresponding ferrocene-betulin conjugates with a 1,2,3-triazole linker.
Similar content being viewed by others
References
Kacprzak, K., Skiera, I., Piasecka, M., and Paryzek, Z., Chem. Rev., 2016, vol. 116, p. 5689. https://doi.org/10.1021/acs.chemrev.5b00302
Xiao, S., Wang, Q., Si, L., Zhou, X., Zhang, Y., Zhang, L., and Zhou, D., Eur. J. Med. Chem., 2016, vol. 124, p. 1. https://doi.org/10.1016/j.ejmech.2016.08.020
Rashid, S., Dar, B.A., Majeed, R., Hamid, A., and Bhat, B.A., Eur. J. Med. Chem., 2013, vol. 66, p. 238. https://doi.org/10.1016/j.ejmech.2013.05.029
Spivak, A.Yu., Gubaidullin, R.R., Galimshina, Z.R., Nedopekina, D.A., and Odinokov, V.N., Tetrahedron, 2016, vol. 72, p. 1249. https://doi.org/10.1016/j.tet.2016.01.024
Spivak, A.Yu., Nedopekina, D.A., Galimshina, Z.R., Khalitova, R.R., Sadretdinova, Z.R., Gubaidullin, R.R., and Odinokov, V.N., Arkivoc, 2018, vol. vii, p. 1. https://doi.org/10.24820/ark.5550190.p010.632
Majeed, R., Sangwan, P.L., Chinthakindi, P.K., Khan, L., Dangroo, N.A., Thota, N., Hamid, A., Sharma, P.R., Saxena, A.K., and Koul, S., Eur. J. Med. Chem., 2013, vol. 63, p. 782. https://doi.org/10.1016/j.ejmech.2013.03.028
Pérez-Labrada, K., Morera, C., Brouard, I., Llerena, R., and Rivera, D.G., Tetrahedron Lett., 2013, vol. 54, p. 1602. https://doi.org/10.1016/j.tetiet.2013.01.058
Chakraborty, B., Dutta, D., Mukherjee, S., Das, S., Maiti, N.C., Das, P., and Chowdhury, C., Eur. J. Med. Chem., 2015, vol. 102, p. 93. https://doi.org/10.1016/j.ejmech.2015.07.035
Govdi, A.I., Vasilevsky, S.F., Nenaidenko, V.G., Sokolova, N.V., and Tolstikov, G.A., Russ. Chem. Bull, Int. Ed., 2011, vol. 60, p. 2401. https://doi.org/10.1007/slll72-011-0369-3
Govdi, A.I., Sorokina, I.V., Baev, D.S., Bryzgalov, A.O., Tolstikova, T.G., Tolstikov, G.A., and Vasilevsky, S.F., Russ. Chem. Bull., Int. Ed., 2015, vol. 64, p. 1327. https://doi.org/10.1007/slll72-015-1013-4
Rodriguez-Hernandez, D., Barbosa, L.C.A., Demuner, A.J., de Almeida, R.M., Fujiwara, R.T., and Ferreira, S.R., Eur. J. Med. Chem., 2016, vol. 124, p. 153. https://doi.org/10.1016/j.ejmech.2016.08.030
Rodriguez-Hernandez, D., Barbosa, L.C.A., Demuner, A.J., Nain-Perez, A., Ferreira, S.R., Fujiwara, R.T., de Almeida, R.M., Heller, L., and Csuk, R., Eur. J. Med. Chem., 2017, vol. 140, p. 624. https://doi.org/10.1016/j.ejmech.2017.09.045
Suman, R., Patel, A., Solano, L., Jampana, G., Gardner, Z.S., Holt, C.M., and Jonnalagadda, S.C., Tetrahedron, 2017, vol. 73, p. 4214. https://doi.org/10.1016/j.tet.2016.ll.056
Bebenek, E., Kaleda-Tomanek, M., Chrobak, E., Latocha, M., and Boryczka, S., Med. Chem. Res., 2018, vol. 27, p. 2051. https://doi.org/10.1007/s00044-018-2213-x
Wei, G., Sun, J., Hou, Z., Luan, W., Wang, S., Cui, S., Cheng, M., and Liu, Y., Eur. J. Med. Chem., 2018, vol. 157, p. 759. https://doi.org/10.1016/j.ejmech.2018.08.036
Khan, I., Guru, S.K., Rath, S.K., Chinthakindi, P.K., Singh, B., Koul, S., Bhushan, S., and Sangwan, P.L., Eur. J. Med. Chem., 2016, vol. 108, p. 104. https://doi.org/10.1016/j.ejmech.2015.ll.018
Antimonova, A.N., Petrenko, N.I., Shakirov, M.M., Rybalova, T.V., Frolova, T.S., Shul’tz, E.E., Kukina, T.P., Sinitsyna, O.I., and Tolstikov, G.A., Chem. Nat. Prod., 2013, vol. 49, p. 657. https://doi.org/10.1007/sl0600-013-0702-l
Thomas, J., Goyvaerts, V., Liekens, S., and Dehaen, W., Chem. Eur. J., 2016, vol. 22, p. 9966. https://doi.org/10.1002/chem.201601928
Snegur, L.V., Babin, V.N., Simenel, A.A., Nekrasov, Yu.S., Ostrovskaya, L.A., and Sergeeva, N.S., Russ. Chem. Bull., Int. Ed., 2010, vol. 59, p. 2167. https://doi.org/10.1007/slll72-014-0756-7
Gasser, G., Ott, I., and Metzler-Nolte, N., J. Med. Chem., 2011, vol. 54, p. 3. https://doi.org/10.1021/jml00020w
Marinero, J.d.J.C., Lapierre, M., Cavailles, V., Saint-Fort, R., Vessieres, A., Top, S., and Jaouen, G., Dalton Trans., 2013, vol. 42, p. 15489. https://doi.org/10.1039/C3DT51917A
Csókas, D., Zupkó, L., Károlyi, B.I., Drahos, L., Holczbauer, T., Palló, A., Czugler, M., and Csámpai, A., J. Organometal. Chem., 2013, vol. 743, p. 130. https://doi.org/10.1016/j.jorganchem.2013.06.040
Panaka, S., Trivedi, R., Jaipal, K., Giribabu, L., Sujitha, P., Kumar, C.G., and Sridhar, B., J. Organomet. Chem., 2016, vol. 813, p. 125. https://doi.org/10.1016/jjorganchem.2016.04.011
Kedge, J.L., Nguyen, H.V., Khan, Z., Male, L., Ismail, M.K., Roberts, H.V., Hodges, N.J., Horswell, S.L., Mehellou, Y., and Tucker, J.H.R., Eur. J. Inorg. Chem., 2017, p. 466. https://doi.org/10.1002/ejic.201600853
El Sayed, A.M.R., El Azab, I.H., and Gobouri, A.A., Monatsh. Chem., 2018, vol. 149, p. 505. https://doi.org/10.1007/s00706-017-2093-7
Anikina, L.V., Shemyakina, D.A., Pavlogradkaya, L.V., Nedugov, A.N., and Glushkov, V.A., Russ. J. Org. Chem., 2014, vol. 50, p. 1180. https://doi.org/10.1134/S1070428014080181
Pavlogradkaya, L.V., Shemyakina, D.A., Eroshenko, D.V., Borisova, I.A., and Glushkov, V.A., Russ. J. Org. Chem., 2018, vol. 54, p. 126. https://doi.org/10.1134/S1070428018010128
Tolstikov, G.A., Goryaev, M.I., Kim, Khya Ok, Khegai, R.A., Zh. Prikl. Khim., 1967, vol. 40, p. 920
Kuznetsov, B.N., Levdanskii, V.A., and Poleszaeva, N.I., Khim. Rast. Syr., 1998, p. 5.
Okamoto, I., Takeya, T., Kagawa, Y., and Kotani, E., Chem. Pharm. Bull., 2000, vol. 48, p. 120. https://doi.org/10.1248/cpb.48.120
Kuznetsova, S.A., Skvortsova, G.P., Malyar, Yu.N., Sokolenko, VA., and Kuznetsov, B.N., Khim. Rast. Syr., 2011, p. 77.
Drebushchak, V.A., Mikhailenko, M.A., Shakhtsneider, T.P., Drebushchak, T.N., Kuznetsova, S.A., and Malyar, Ju.N., J. Ther Anal. Calorim., 2014, vol. 115, p. 2521. https://doi.org/10.1007/sl0973-013-3578-l
Levdanskii, V.A., Levdanskii, A.V., and Kuznetsov, B.N., Chem. Nat. Prod., 2017, vol. 53, p. 310. https://doi.org/10.1007/sl0600-017-1976-5
Yang, S.-J., Liu, M.-C., Xiang, H.-M., Zhao, Q., Xue, W., and Yang, S., Eur. J. Med. Chem., 2015, vol. 102, p. 249. https://doi.org/10.1016/j.ejmech.2015.08.004
Qian, K., Yu, D., Chen, C.-H., Huang, L., Morris-Natschke, S.L., Nitz, T.J., Salzwedel, K., Reddick, M., Allaway, G.P., and Lee, K.-H., J. Med. Chem., 2009, vol. 52, p. 3248. https://doi.org/10.1021/jm900136j
Sun, I.-C., Wang, H.-K., Kashiwada, Y., Shen, J.-K., Cosentino, L.M., Chen, C.-H., Yang, L.-M., and Lee, K.-H., J. Med. Chem., 1998, vol. 41, p. 4648. https://doi.org/10.1021/jm980391g
Creary, X., Anderson, A., Brophy, C., Crowell, F., and Funk, Z., J. Org. Chem., 2012, vol. 77, p. 8756. https://doi.org/10.1021/jo301265t
Mosmann, T., J. Immunol. Methods, 1983, vol. 65, p. 55. https://doi.org/10.1016/0022-1759(83)90303-4
Polin, J. and Schottenberger, H., Org. Synth., Coll., 1996, vol. 73, p. 262. https://doi.org/10.15227/orgsyn.073.0262
CrysAlisPro, Version 1.171.37.33, Agilent Technologies (release 27-03-2014 CrysAlisl71.NET).
Palatinus, L. and Chapuis, G., J. Appl. Cryst., 2007, vol. 40, p. 786. https://doi.org/10.1107/S0021889807029238
Sheldrick, G.M., Acta Cryst., 2015, vol. C71, p. 3. https://doi.org/10.1107/S2053229614024218
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., and Puschmann, H., J. Appl. Cryst., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Acknowledgments
The authors express their gratitude to Engineer I.A. Borisova for measuring the IR spectra, Leading Engineer O.A. Maiorova for measuring the 1H and 13C NMR spectra, and Scientific Researcher A.V. Kharitonova for performing elemental analysis.
Funding
The work was financially supported by the Russian Foundation for Basic Research (project no. 16-33-00147) and the "Basic Science for Medicine" Complex Program of the Ural Branch of the Russian Academy of Sciences (project no. 18-7-3-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Russian Text © The Author(s), 2019, published in Zhurnal Organicheskoi Khimii, 2019, Vol. 55, No. 11, pp. 1722–1729.
Rights and permissions
About this article
Cite this article
Glushkov, V.A., Shemyakina, D.A., Zhukova, N.K. et al. Ferrocenyltriazoles from 3β,28-Diacylbetulin: Synthesis and Cytotoxic Activity. Russ J Org Chem 55, 1690–1697 (2019). https://doi.org/10.1134/S1070428019110083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019110083