Abstract
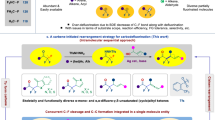
A highly environmentally friendly procedure was developed for the Knoevenagel condensation of aromatic aldehydes with diethyl malonate in the presence of FeCl3 and N-fluorobenzenesulfonimide as a source of electrophilic fluorine under solvent-free conditions. The scope of the reaction was explored using commercially available substrates. The reaction with substituted salicylaldehydes afforded the corresponding coumarin derivatives which attract interest due to their potential medicinal importance.
Similar content being viewed by others
References
Jones, G., Org. React., 1967, vol. 15, p. 204
Ap-Simon, J.W., Hooper, J.W., and Laishes, B.A., Can. J. Chem., 1970, vol. 48, p. 3064
Van der Baan, J.L. and Bickelhaupt, F., Tetrahedron, 1974, vol. 30, p. 2447
Tanaka, M., Oota, O., Hiramatsu, H., and Fujiwara, K., Bull. Chem. Soc. Jpn., 1988, vol. 61, p. 2473
Tietze, L.F. and Beifuss, U., Comprehensive Organic Synthesis. Selectivity, Strategy, and Efficiency in Modern Organic Chemistry, Trost, B.M. and Fleming, I., Eds., Oxford: Pergamon, 1991, vol. 2, p. 341
Kim, J.K., Kwon, P.S., Kwon, T.W., Chung, S.K., and Lee, J.W., Synth. Commun., 1996, vol. 26, p. 535
Ren, Z., Cao, W., and Tong, W., Synth. Commun., 2002, vol. 32, p. 3475
Bigi, F. and Quarantelli, C., Curr. Org. Synth., 2012, vol. 9, p. 31
Heravi, M.M., Asadi, S., and Azarakhshi, F., Curr. Org. Synth., 2014, vol. 11, p. 701
Song, H.Y., Jin, R.H., Jin, F.X., Kang, M.R., Li, Z., and Chen, J., Catalysts, 2016, vol. 6, p. 106.
Prout, F.S., J. Org. Chem., 1953, vol. 18, p. 928
Lelean, P.M. and Morris, J.A., Chem. Commun., 1968, p. 239
Lehnert, W., Synthesis, 1974, p. 667
Texier-Boullet, F. and Foucaud, A., Tetrahedron Lett., 1982, vol. 23, p. 4927
Tanikaga, R., Konya, N., and Kaji, A., Chem. Lett., 1985, vol. 26, vol. 14, p. 1583
Prajapati, D. and Sandhu, J.S., Chem. Lett., 1992, vol. 21, p. 1945
Macquarrie, D.J., Clark, J.H., Jackson, D.B., Lambert, A., Mdoe, J.E.G., and Priest, A., Spec. Publ. R. Soc. Chem., 1998, vol. 216, p. 174
Narsaiah, V. and Nagaiah, D.K., Synth. Commun., 2003, vol. 33, p. 3825
Yadav, J.S., Reddy, B.V.S., Basak, A.K., Visali, B., Narsaiah, A.V., and Nagaiah, K., Eur. J. Org. Chem., 2004, p. 546
Younes, M. and Ridha, B.S., C. R. Chim., 2007, vol. 10, p. 1162
Fraile, J.M., Garc, N., Herrer, C.I., and Mayoral, J.A., Catal. Sci. Technol., 2013, vol. 3, p. 436
Gao, S.Q., Xiao, D., Yang, Y., Wei, X.Y., Sun, S., Lang, J., and Lv, C.W., Heterocycles, 2016, vol. 92, p. 1698
Devi, R., Begum, P., Bharali, P., and Deka, R.C., ACS Omega, 2018, vol. 3, p. 7086.
Snider, B.B. and Lu, Q., J. Org. Chem., 1996, vol. 61, p. 2839
Fukuyama, T. and Liu, G., J. Am. Chem. Soc., 1996, vol. 118, p. 7426
Nicolaou, K.C., Xu, J.Y., Kim, S., Ohshima, T., Hosokawa, S., and Pfefferkorn, J., J. Am. Chem. Soc., 1997, vol. 119, p. 11353
Tietze, L.F. and Zhou, Y., Angew. Chem., Int. Ed., 1999, vol. 38, p. 2045
Tietze, L.F. and Modi, A., Med. Res. Rev., 2000, vol. 20, p. 304
Boucard, V., Macromolecules, 2001, vol. 34, p. 4308
Siddegowda, K.S., Zabiulla, K.M., and Yellappa, S., Org. Commun., 2016, vol. 9, p. 119
Vellalacheruvu, R., Leela, R.S., and Ravindranath, L.K., Int. J. Org. Chem., 2017, vol. 7, p. 269
Kumbar, S.S. and Hosamani, K.M., ChemistrySelect, 2018, vol. 3, p. 5678
Riveira, M.J., Marcarino, M.O. and La-Venia, A., Org. Lett., 2018, vol. 20, p. 4000
Levchenko, K.S., Chudov, K.A., Zinoviev, E.V., Lyssenko, K.A., Demin, D.U., Poroshin, N.O., Shmelin, P.S., and Grebennikov, E.P., Tetrahedron Lett., 2018, vol. 59, p. 2788.
Bogdal, D., Stepien, I., and Pajda, M., Pol. J. Chem. Technol., 2003, vol. 5, p. 90
Karade, N.N., Gampawar, S.V., Shinde, S.V., and Jadhav, W.N., Chin. J. Chem., 2007, vol. 25, p. 1686
Majid, M.H., Parvaneh, A., Mina, S., Narges, K., and Niloofar, T.H., Bull. Chem. Soc. Ethiop., 2011, vol. 25, p. 315
Pedro, V., Francisco, S., and Emilia, T., Molecules, 2011, vol. 16, p. 4379
Sunanda, B.P. and Ganapati, S.S., Environ. Chem. Lett., 2012, vol. 10, p. 363
He, X.W., Yan, Z.L., Hu, X.Q., Zuo, Y., Jiang, C., Jin, L., and Shang, Y.J., Synth. Commun., 2014, vol. 44, p. 1507
Angelo, V., Lucia, F., Giulia, M., Sebastiano, A., Luca, G., and Alessandra, T., Tetrahedron, 2014, vol. 70, p. 6781
Vekariya, R.H. and Patel, H.D., Synth. Commun., 2014, vol. 44, p. 2756
Mudulkar, S., Gannerla, S., and Bhimapaka, C.R., Tetrahedron Lett., 2015, vol. 56, p. 1338
Mona, H.S. and Sepideh, N.D., J. Catal., 2016, vol. 6, p. 423
Gonzalez-Gonzalez, A., Aparicio-Solano, D.M., Aguilar-Mariscal, H., Gomez-Rivera, A., Roa, L.F., Alvarado-Sanchez, C., Lobato-Garcia, C.E., and Romero-Ceronio, N., Am. J. Org. Chem., 2016, vol. 6, p. 17
Abdul Rahman, F.S., Yusufzai, S.K., Osman, H., and Mohamad, D., J. Phys. Sci., 2016, vol. 27, p. 77
Danish, K., Sayeed, M., Meshari, A.A., Mohammed, I.A., and Naseem, A., Tetrahedron Lett., 2017, vol. 58, p. 3183
Syed, S.S., Amir, A.K., Misbahul, A.K., Shanti, F.S., Mohd, S.J., and Pang, S.C., Asian J. Chem., 2017, vol. 29, p. 261
Salem, M.A., Helal, M.H., Gouda, M.A., Ammar, Y.A., El-Gaby, M.S.A., and Abbas, S.Y., Synth. Commun., 2018, vol. 48, p. 1534
Heravi, M.M., Zadsirjan, V., Dehghani, M., and Ahmadi, T., Tetrahedron, 2018, vol. 74, p. 3391
Kumbar, S.S. and Hosamani, K.M., ChemistrySelect, 2018, vol. 3, p. 5678
Kolita, S., and Bhuyan, P.J., ChemistrySelect, 2018, vol. 3, p. 1411.
Differdin, E. and Ofner, H., Synlett, 1991, no. 3, p. 187
Davis, F.A. and Reddy, R.E., Tetrahedron: Asymmetry, 1994, vol. 5, p. 955
Ma, J. and Cahard, D., Chem. Rev., 2004, vol. 104, p. 6119
Ma, J.A., and Cahard, D., Tetrahedron: Asymmetry, 2004, vol. 15, p. 1007
Zhang, Y., Shibatomi, K., and Yamamoto, H., Synlett, 2005, no. 18, p. 2837
Giovanelli, E., Doris, E., and Rousseau, B., Tetrahedron Lett., 2006, vol. 47, p. 8457
Baudoux, J. and Cahard, D., Org. React., 2007, vol. 69, p. 347
Sibbald, P.A. and Rosewall, C.F., J. Am. Chem. Soc., 2009, vol. 131, p. 15945
Engle, K.M., Mei, T.S., Wang, X., and Yu, J.Q., Angew. Chem., Int. Ed., 2011, vol. 50, p. 1478
Ball, N.D., Gary, J.B., Ye, Y., and Sanford, M.S., J. Am. Chem. Soc., 2011, vol. 133, p. 7577
Wang, X., Leow, D., and Yu, J.Q., J. Am. Chem. Soc., 2011, vol. 133, p. 13864
Xu, T. and Liu, G., Org. Lett., 2012, vol. 14, p. 5416
Barker, T.J. and Boger, D.L., J. Am. Chem. Soc., 2012, vol. 134, p. 13 588
Liang, T. and Neumann, C.N., Angew. Chem., Int. Ed., 2013, vol. 52, p. 8214
Zhang, K., Zhao, G., and Cao, W., Tetrahedron., 2014, vol. 70, no. 35, p. 5659
Li, Y. and Zhang, Q., Synthesis, 2015, vol. 47, p. 159.
Du, C., Wang, X.Y., Jin, S.F., Shi, H., Li, Y.M., Pang, Y.D., Liu, Y., Cheng, M.S., Guo, C., and Liu, Y.X., Asian J. Org. Chem., 2016, vol. 5, p. 755.
Xie, F.K., Du, C., Pang, Y.D., Lian, X., Xue, C.T., Chen, Y.Y., Wang, X.F., Cheng, M.S., Guo, C., Lin, B., and Liu, Y.X., Tetrahedron Lett., 2016, vol. 57, p. 5820.
Shi, H., Du, C., Zhang, X.H., Xie, F.K., Wang, X.Y., Cui, S.S., Peng, X.S., Cheng, M.S., Lin B., and Liu, Y.X., J. Org. Chem., 2018, vol. 83, p. 1312.
Rousseau, O., Delaunay, T., and Robiette, R., Synlett, 2014, vol. 25, p. 519.
Rasna, D., Pakiza, B., Pankaj, B., and Ramesh, C.D., ACS Omega, 2018, vol. 3, p. 7086.
Che, J. and Lam, Y., Synlett, 2010, no. 16, p. 2415.
Silver, R.F., Kerr, K.A., Frandsen, P.D., Kelley, S.J., and Holmes, H.L., Can. J. Chem., 1967, vol. 45, p. 1001.
Rukachaisirikul, Y.S.V. and Kaeobamrung, J., Tetrahedron Lett., 2017, vol. 58, p. 168.
Booher, R.N., Smits, S.E., Turner, W.W., and Pohland, A., J. Med. Chem., 1977, vol. 20, p. 7885.
Konidena, L.N.S., Chettu, S.K., Sorra, K., Valluru, K.R., Rao, N.S.K., Babu, K.R., and Guduru, R., Tetrahedron Lett., 2017, vol. 58, p. 1127.
Macdonald, D.I. and Durst, T., J. Org. Chem., 1988, vol. 53, p. 3663.
Shrivastava, S.K., Srivastava, P., Bandresh, R., Tripathi, P.N., and Tripathi, A., Bioorg. Med. Chem., 2017, vol. 25, p. 4424.
Khan, D., Mukhtar, S., Alsharif, A.M., Alahmdi, M.I., and Ahmed, N., Tetrahedron Lett., 2017, vol. 58, p. 3183.
Liu, Q., Zhu, F.P., Jin, X.L., Wang, X.J., Chen, H., and Wu, L.Z., Chem. Eur. J., 2015, vol. 21, p. 10326.
He, X.W., Shang, Y.J., Zhou, Y., Yu, Z.Y., Han, G., Jin, W.J., and Chen, J.J., Tetrahedron, 2015, vol. 71, p. 863.
Latha, R.N., Prashanth, T., Sridhar, M.A., Ranganatha, V.L., Shaukath, A.K., and Lokanath, N.K., Mol. Cryst. Liq. Cryst., 2015, vol. 623, p. 265.
Paul, K., Bindal, S., and Luxami, V., Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 3667.
Chilin, A., Battistutta, R., Bortolato, A., Cozza, G., Zanatta, S., Poletto, G., Mazzorana, M., Zagotto, G., Uriarte, E., Guiotto, A., Pinna, L.A., Meggio, F., and Moro, S., J. Med. Chem., 2008, vol. 51, p. 752.
da Silveira Pinto, L.S. and de Souza, M.V.N., Synthesis, 2017, vol. 49, p. 2677.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interests
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Yang, L., Zhu, J., Xie, F. et al. Solvent-Free FeCl3-Assisted Electrophilic Fluorine-Catalyzed Knoevenagel Condensation to Yield α,β-Unsaturated Dicarbonyl Compounds and Coumarins. Russ J Org Chem 55, 1053–1060 (2019). https://doi.org/10.1134/S1070428019070236
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019070236