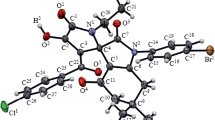
Abstract
Reaction with phenylhydrazine of 3Н-pyrroles spirobound to a furan ring, N-acylimino-substituted 2-oxa-7-azaspiro[4.4]nona-3,6,8-trienes, occurs with a diasteroselective formation of previously unknown derivatives of 1,2,4-triazole: 5-amino-3-(5-alkyl-1-phenyl-1H-1,2,4-triazol-3-yl)-2-morpholin-4-yl-3H-pyrrole-4-carbonitriles.
Similar content being viewed by others
References
Küçükgüzel, Ş.G. and Çıkla-Süzgün, P., Eur. J. Med. Chem., 2015, vol. 97, p. 830.
Maddila, S., Pagadala, R., and Jonnalagadda, S.B., Lett. Org. Chem., 2013, vol. 10, p. 693.
Kaur, R., Dwivedi, A.R., Kumar, B., and Kumar, V., Anticancer Agents Med. Chem., 2016, vol. 16, p. 465.
Kamboj, V.K., Verma, P.K., Dhanda, A., and Ranjan, S., Cent. Nerv. Syst. Agents Med. Chem., 2015, vol. 15, p. 17.
Kochikyan, T.V., Samvelyan, M.A., Arutyunyan, E.V., Arutyunyan, V.S., Avetisyan, A.A., Malakyan, M.G., Vardevanyan, L.A., and Badzhinyan, S.A., Pharm. Chem. J., 2011, vol. 44, p. 525. doi 10.1007/s11094-011-0509-y
Gençer, H.K., Çevik, U.A., Levent, S., Saglýk, B.N., Korkut, B., Özkay, Y., Iigin, S., and Öztürk, Y., Molecules, 2017, vol. 22, p. 507.
Al-Soud, Y.A., Marchais-Oberwinkler, S., Frotscher, M., and Hartmann, R.W., Arch. Pharm., 2012, vol. 345, p. 610.
Yang, L.P., Keam, S.J., and Keating, G.M., Drugs, 2007, vol. 67, p. 2211.
Salehi, S., Saljooghi, A.Sh., and Shiri, A., Eur. J. Pharm., 2016, vol. 781, p. 209.
Belikov, M.Yu., Ershov, O.V., Lipovskaya, I.V., Fedoseev, S.V., and Nasakin, O.E., Russ. J. Org. Chem., 2013, vol. 49, p. 864. doi 10.1134/S1070428013060110
Ershov, O.V., Nasakin, O.E., and Belikov, M.Yu., RF Patent no. 2475489, 2013.
Belikov, M.Yu., Ievlev, M.Yu., Belikova, I.V., Ershov, O.V., Tafeenko, V.A., and Surazhskaya, M.D., Chem. Heterocycl. Compd., 2015, vol. 51, p. 518. doi 10.1007/s10593-015-1731-4
Belikov, M.Yu., Ershov, O.V., Lipovskaya, I.V., Fedoseev, S.V., Lipin, K.V., and Nasakin, O.E., Russ. J. Org. Chem., 2013, vol. 49, p. 1195. doi 10.1134/S1070428013080162
Belikov, M.Yu., Belikova, I.V., Ershov, O.V., Fedoseev, S.V., and Nasakin, O.E., Russ. J. Org. Chem., 2016, vol. 52, p. 1312. doi 10.1134/S1070428016090104
Padmavathi, V., Radha, L.T., Mahesh, K., and Padmaja, A., Chem. Pharm. Bull., 2009, vol. 57, p. 1200.
Cirrincione, G., Almerico, A.M., Grimaudo, S., Diana, P., Mingoia, F., Barraja, P., and Misuraca, F., Farmaco, 1996, vol. 51, p. 49.
Sheldrick, G.M., Acta Cryst., 2008, vol. A64, p. 112.
Brandenburg, K., DIAMOND, Release 2.1d, Bonn, Germany: Crystal Impact, 2000.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.Yu. Belikov, I.V. Belikova, O.V. Ershov, S.V. Fedoseev, V.A. Tafeenko, 2017, published in Zhurnal Organicheskoi Khimii, 2017, Vol. 53, No. 11, pp. 1659–1663.
Rights and permissions
About this article
Cite this article
Belikov, M.Y., Belikova, I.V., Ershov, O.V. et al. N-acylimino-substituted 2-oxa-7-azaspiro[4.4]nona-3,6,8-trienes in the synthesis of 3-(1H-1,2,4-triazol-3-yl)-3H-pyrrole-4-carbonitriles. Russ J Org Chem 53, 1696–1700 (2017). https://doi.org/10.1134/S107042801711015X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042801711015X