Abstract
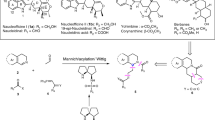
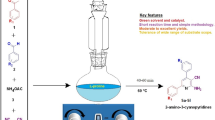
Aspartic acid was found to be an efficient organocatalyst for the one-pot synthesis of tri- and tetrasubstituted imidazoles under solvent-free conditions. The remarkable advantages offered by this method are the inexpensive and green catalyst, mild reaction conditions, simple procedures, and excellent yield of products.
Similar content being viewed by others
References
Lambardino, J.G. and Wiseman, E.H., J. Med. Chem., 1974, vol. 17, p. 1182.
Uçucu, U., Karaburun, N.G., and Işikdağ, I., Il Farmaco, 2001, vol. 56, p. 285.
Yesilada, A., Koyunoglu, S., Saygili, N., Kupeli, E., Yesilada, E., Bedir, E., and Khan, I., Arch. Pharm. Pharm. Med. Chem., 2004, vol. 337, p. 96.
Quattara, L., Debaert, M., and Cavier, R., Il Farmaco, 1987, vol. 42, p. 449.
Dutta, S., Mariappan, G., Roy, S., and Verma, M., Indian Drugs, 2009, vol. 46, p. 50.
Sengupta, A.K. and Bhattacharya, T., J. Indian Chem. Soc., 1983, vol. 60, p. 373.
Navidpour, L., Shadnia, H., Shafaroodi, H., Amini, M., Dehpour, A.R., and Shafiee, A., Bioorg. Med. Chem., 2007, vol. 15, p. 1976.
Fluoret, G., J. Med. Chem., 1970, vol. 13, p. 843.
Brogden, R.N., Heel, R.C., Speight, T.M., and Avery, G.S., Drugs, 1978, vol. 16, p. 387.
Brimblecombe, R.W., Duncan, W.A.M., Durant, G.J., Emmett, J.C., Ganellin, C.R., and Parsons, M.E., J. Int. Med. Res., 1975, vol. 3, p. 86.
Hunkeler, W., Mohler, H., Pieri, L., Polc, P., Bonetti, E.P., Cumin, R., Schaffner, R., and Haefely, W., Nature, 1981, vol. 290, p. 514.
Murry, J.A., Curr. Opin. Drug Discovery Dev., 2003, vol. 6, p. 945.
Murthy, S.M., Madhav, B., and Nageswar, Y.V.D., Tetrahedron Lett., 2010, vol. 51, p. 5252.
Schubert, H. and Stodolka, H., J. Prakt. Chem., 1963, vol. 22, p. 130.
Tsuji, J., Sakai, K., Nemoto, H., and Nagashima, H., J. Mol. Catal., 1983, vol. 18, p. 169.
Frantz, E.D., Morency, L., Soheili, A., Murry, J.A., Grabowski, E.J.J., and Tillyer, R.D., Org. Lett., 2004, vol. 6, p. 843.
Japp, F.R. and Robinson, H.H., Ber., 1882, vol. 15, p. 1268.
Wasserman, H.H., Long, Y.O., Zhang, R., and Parr, J., Tetrahedron Lett., 2002, vol. 43, p. 3351.
Liu, J., Chem, J., Zhao, J., Zhao, Y., Li, L., and Zhang, H., Synthesis, 2003, p. 2661.
Weinmann, H., Harre, M., Koeing, K., Merten, E., and Tilestam, U., Tetrahedron Lett., 2002, vol. 43, p. 593.
Sarshar, S., Siev, D., and Mjalli, A.M.M., Tetrahedron Lett., 1996, vol. 37, p. 835.
Tshoar, R.H., Jahanbakhshi, H., Mousavizadeh, F., and Rahnamafar, R., Gazi Univ. J. Sci., 2012, vol. 25, p. 29.
Ghorbani-Choghamarani, A. and Norouzi, M., Bull. Korean Chem. Soc., 2011, vol. 32, p. 1399.
Ghorbani-Choghamarani, A., Zolfigol, M.A., Hajjami, M., Darvishi, K., and Gholamnia, L., Collect. Czech. Chem. Commun., 2010, vol. 75, p. 607.
Ghorbani-Choghamarani, A. and Zeinivand, J., J. Iran. Chem. Soc., 2010, vol. 7, p. 190.
Ghorbani-Choghamarani, A., Zolfigol, M.A., and Rastegar, T., Chin. J. Catal., 2009, vol. 30, p. 273.
Ghorbani-Choghamarani, A., Hajjami, M., Goudarziafshar, H., Nikoorazm, M., Mallakpour, S., Sadeghizadeh, F., and Azadi, G., Monatsh. Chem., 2009, vol. 140, p. 607.
Ghorbani-Choghamarani, A. and Taghipour, T., Lett. Org. Chem., 2011, vol. 8, p. 470.
Ghorbani-Choghamarani, A. and Azadi, G., J. Iran. Chem. Soc., 2011, vol. 8, p. 1082.
Ghorbani-Choghamarani, A., Nikoorazm, M., and Azadi, G., J. Serb. Chem. Soc., 2012, vol. 77, p. 173.
Mohammadi, A., Keshvari, H., Sandaroos, R., Maleki, B., Rouhi, H., Moradi, H., Sepehr, Z., and Damavandi, S., Appl. Catal. A, 2012, vol. 73, p. 73.
Bahrami, K., Khodaei, M.M., and Nejati, A., Monatsh. Chem., 2011, vol. 142, p. 159.
Montazeri, N., Pourshamsian, K., Khoddadi, M., and Khoddadi, K., Orient. J. Chem., 2011, vol. 27, p. 1023.
Sadeghi, B., Mirjalili, B.B.F., and Hashemi, M.M., Tetrahedron Lett., 2008, vol. 49, p. 2575.
Nagarapu, L., Apuri, S., and Kantevari, S., J. Mol. Catal. A: Chem., 2007, vol. 266, p. 104.
Wang, X.B., He, L., Jian, T.Y., and Ye, S., Chin. Chem. Lett., 2012, vol. 23, p. 13.
Pandit, S.S., Bhalerao, S.K., Aher, U.S., Adhav, G.L., and Pandit, V.U., J. Chem. Sci., 2011, vol. 123, p. 421.
Wang, X.C., Gong, H.P., Quan, Z.J., Li, L., and Ye, H.L., Chin. Chem. Lett., 2009, vol. 20, p. 44.
Bhosale, S.V., Kalyankar, M.B., Nalage, S.V., Bhosale, D.S., Pandhare, S.L., Kotbagi, T.V., Umbarkar, S.B., and Dongare, M.K., Synth. Commun., 2011, vol. 41, p. 762.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Ghorbani-Choghamarani, A., Hajjami, M., Gholamian, F. et al. Aspartic acid as a highly efficient and nontoxic organocatalyst for the one-pot synthesis of tri- and tetrasubstituted imidazoles under solvent-free conditions. Russ J Org Chem 51, 352–356 (2015). https://doi.org/10.1134/S1070428015030100
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428015030100