To the memory of Professor G.I. Koldobskii
Abstract
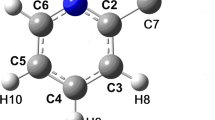
It was shown by the method of powder X-ray diffraction analysis that in the crystalline state the product of the reaction of thiourea with chloroacetic acid in water, (carbamimidoylsulfanyl)acetic acid, existed in the zwitter-ion tautomeric form. The structure consists of virtually planar infi nite layers normal to the c axis of the unit cell which are bound by van der Waals interactions. The layers are formed by infi nite rows elongated along the b axis of the unit cell consisting of materially planar zwitter-ionic molecules linked by strong bifurcated hydrogen bonds. The results of quantum-chemical calculations by PM6 method are in agreement with the XRD results: whereas an isolated molecule exists in nonzwitter-ionic tautomeric form, in the crystal only the zwitterionic tautomer is present.
Similar content being viewed by others
References
Maly, R. Lieb. Ann. 1877, vol. 189, p. 380.
Ray, P.C. and Fernandes, F.V. J. Chem. Soc., 1914, vol. 105, p. 2159.
Desai, R.D., Hunter, R.F., and Koppar, L.G., Rec. Trav. Chim., 1935, vol. 54, p. 118.
Lazarev, D.B., Ramsh, S.M., and Ivanenko, A.G., Zh. Org. Khim., 2000, vol. 70, p. 475.
Allen, F.H., Kennard, O., Watson, D.G., Brammer, L., Orpen, A. G., and Taylor, R., J. Chem. Soc., Perkin Trans. II, 1987, S1.
Brand, H., Hubrich, C., Polborn, K., Schulz, A., and Villinger, A., Acta Cryst., Sect. E: Struct. Rep. Online., 2007, 63, no. 12, o4733.
Kushakova, P.M., Ramsh, S.M., and Garabadzhiu, A.V., Khim. Geterotsikl. Soedin., 2006, p. 250.
Roothaan, C.C., J. Rev. Mod. Phys., 1951, vol. 23, no. 2, p. 69.
Stewart, J.J.P., J. Mol. Model., 2007, vol. 13, p. 1173.
Mulliken, R.S., J. Chem. Phys., 1955, vol. 23, p. 1833.
Pal’m, V.A., Vvedenie v teoreticheskuyu organicheskuyu khimiyu (Introduction on Theoretical Organic Chemistry), Moscow: Vysshaya Shkola, 1974, p. 168.
Altomare, A., Camalli, M., Cuocci, C., Giacovazzo, C., Moliterni, A., and Rizzi, R., J. Appl. Cryst., 2009, vol. 42, p. 1197.
Macrae, C.F., Bruno, I.J., Chisholm, J.A., Edgington, P.A., McCabe, P., Pidcock, E., Rodrigues-Monge, L., Taylor, R., van de Streek, J., and Wood, P.A., J. Appl. Cryst., 2008, vol. 41, p. 466.
Stewart, J.J.P., Program package MOPAC2009. http://OpenMOPAC.net
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguyen, K.A., Su, S., Windus, T.L., Dupuis, M., and Montgomery, J.A., J. Comput. Chem., 1993, vol. 14, p. 1347; http://molecularmodelingbasics.blogspot.com/search/label/gamess.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original English Text © V.S. Fundamenskii, S.M. Ramsh, V.A. Brouskov, A.V. Smirnova, A.Yu. Yanichev, M. B. Fleisher, S.V. Belyakov, 2013, published in Zhurnal Organicheskoi Khimii, 2013, Vol. 49, No. 5, pp. 690–696.
Rights and permissions
About this article
Cite this article
Fundamenskii, V.S., Ramsh, S.M., Brouskov, V.A. et al. Study of the structure of (carbamimidoylsulfanyl)acetic (“pseudothiohydantoic”) acid by XRD and PM6 methods. Russ J Org Chem 49, 672–677 (2013). https://doi.org/10.1134/S1070428013050060
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428013050060