Abstract
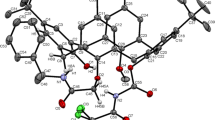
By sulfonylation of tetra(p-tert-butyl)-27-propoxy-25-[N-(1-phenylethyl)carbamoylmethoxy]calix[4] arene diastereomeric inherently chiral calixarenes with the ABCH substitution at the lower rim were synthesized and separated by column chromatography. The alkylation of these compounds afforded the corresponding calixarenes with the ABCD substitution type. The absolute configuration of compounds was established by XRD analysis.
Similar content being viewed by others
References
Gutsche, C.D., Calixarenes: An Introduction, Cambridge: RSCPublishing, 2008.
Kubo, Y., Maeda, S., Tokita, S., and Kubo, M., Nature., 1996, vol. 382, 522; Zheng, Y.-S., Zhang, C. Org. Lett., 2004, vol. 6, 1189; Liu, X.-X. and Zheng, Y.-S., Tetrahedron Lett., 2006, vol. 47, p. 6357.
Shirakawa, S., Moriyama, A., and Shimizu, S., Org. Lett., 2007, vol. 9, p. 3117; Xu, Z.-X., Li, G.-K., Chen, Ch.-F., and Huang, Z.-T., Tetrahedron., 2008, vol. 64, p. 8668.
Steyer, S., Jeunesse, C., Harrowfield, J., and Matt, D., J. Chem. Soc., Dalton Trans., 2005, p. 1301; Gaeta, C., De, Rosa, M., Fruilo, M., Soriente, A., and Neri, P., Tetrahedron: Asymmetry., 2005, vol. 16, p. 2333.
Luo, J., Zheng, Q.-Y., Chen, C.-F., and Huang, Z.-T., Tetrahedron, 2005, vol. 61, p. 8517; Karakucuk, A., Durmaz, M., Sirit, A., Yilmaz, M., and Demir, A.S., Tetrahedron: Asymmetry., 2006, vol. 17, p. 1963.
Narumi, F., Suzuki, T., Onodera, T., and Miyano, S., Enantiomer., 2000, vol. 5, p. 83; Krawinkler, K.H., Maier, N.M., Ungaro, R., Sansone, F., Casnati, A., and Lindner, W., Chirality, 2003, vol. 15, p. 17.
He, Y., Xiao, Y, Meng, L., Zeng, Z., Wu, X., and Wu, C.-T., Tetrahedron Lett., 2002, vol. 43, p. 6249; Narumi, F., Hattori, T., Matsumura, N., Onodera, T., Katagiri, H., Kabuto, C., Kameyama, H., and Miyano, S., Tetrahedron, 2004, vol. 60, p. 7827.
Li, S.-Y., Xu, Y.-W., Liu, J.-M., and Su, Ch.-Y., Int. J. Mol. Sci., 2011, vol. 12, p. 429.
Dieleman, C., Steyer, S., Jeunesse, C., and Matt, D., J. Chem. Soc., Dalton Trans., 2001, p. 2508; Narumi, F., Hattori, T., Yamabuki, W., Kabuto, C., and Kameyama, H., Tetrahedron: Asymmetry, 2005, vol. 16, p. 793; Boyko, V.I., Shivanyuk, A., Pyrozhenko, V.V., Zubatyuk, R.I., Shiskin, O.V., and Kalchenko, V.I., Tetrahedron Lett., 2006, vol. 47, p. 7775; Yakovenko, A.V., Boyko, V.I., Danylyuk, O., Suwinska, K., Lipkowski, J., and Kalchenko, V.I., Org. Lett., 2007, vol. 9, p. 1183; Xu, Z.-X., Zhang, Ch., Zheng, Q.-Y., Chen, Ch.-F., and Huang, Z.-T., Org. Lett., 2007, vol. 9, p. 4447; Xu, Z.-X., Huang, Z.-T., and Chen, Ch.-F., Tetrahedron Lett., 2009, vol. 50, p. 5430; Boyko, V.I., Matvieiev, Yu.I., Klyachina, M.A., Yesypenko, O.A., Shishkina, S.V., Shishkin, O.V., and Kalchenko, V.I., Tetrahedron, 2009, vol. 65, p. 4220.
Caccamese, S., Bottino, A., Cunsolo, F., Parlato, S., and Neti, P., Tetrahedron: Asymmetry, 2000, vol. 11, p. 3103; Hesek, D., Inoue, Y., Drew, M.G., Beer, P.D., Hembury, G.A., Ishida, H., and Aoki, F., Org. Lett., 2000, vol. 2, p. 2237; Tairov, M.A., Vysotsky, M.O., Kalchenko, O.I., Pyrozhenko, V.V., and Kalchenko, V.I., J. Chem. Soc., Perkin Trans. I, 2002, p. 1405; Cao, Y.-D., Luo, J., Zheng, Q.-Y., Chen, C.-F., Wang, M.-X., and Huang, Z.-T., J. Org. Chem., 2004, vol. 69, p. 206; Miao, R., Zheng, Q.-Y., Chen, C.-F., and Huang, Z.-T., J. Org. Chem., 2005, vol. 70, p. 7662; Talotta, C., Gaeta, C., Troisi, F., Monaco, G., Zanasi, R., Mazzeo, G., Rosini, C., and Neri, P., Org. Lett., 2010, vol. 12, p. 2912.
Kliachyna, M.A., Yesypenko, O.A., Pirozhenko, V.V., Shishkina, S.V., Shishkin, O.V., Boyko, V.I., and Kalchenko, V.I., Tetrahydron, 2009, vol. 65, p. 7085.
Esypenko, O.A., Kliachina, M.A., Boyko, V.I., and Kalchenko, V.I., Vopr. Khim. Kkhim. Tekhnol., 2009, no. 4, p. 10.
Böhmer, V., Angew. Chem., Int. Ed., 1995, vol. 34, p. 713; Iwamoto, K., Araki, K., and Shinkai, S., J. Org. Chem., 1991, vol. 56, p. 4955.
Jaime, C., de Mendoza, J., Prados, P., Nieto, P.M., and Sanchez, C., J. Org. Chem., 1991, vol. 56, p. 3372.
Sheldrick, G.M., Acta Cryst. A, 2008, vol. 64, p. 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.A. Yesypenko, V.I. Boyko, O.V. Shishkin, S.V. Shishkina, V.V. Pirozhenko, V.I. Kalchenko, 2012, published in Zhurnal Organicheskoi Khimii, 2012, Vol. 48, No. 2, pp. 292–299.
Rights and permissions
About this article
Cite this article
Yesypenko, O.A., Boyko, V.I., Shishkin, O.V. et al. Synthesis and stereochemical configuration of diastereomeric inherently chiral calixarenes with ABCH and ABCD type of substitution at the lower rim. Russ J Org Chem 48, 284–292 (2012). https://doi.org/10.1134/S1070428012020200
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428012020200