Abstract
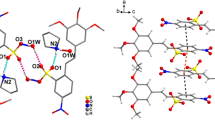
X-Ray diffraction analysis was performed of 1-amino-X-pyrazinium mesitylenesulfonates (X=H, 2-NH2, 3-NHCOMe, 3-OMe, 3-Cl). In all events save 1,2-diaminopyrazinium cation the bond length of N-NH2 was shorter than that of N-N bond but considerably longer than the length of the double bond N=N. In the 1,2-diaminopyrazinium cation the bond distance C2-NH2 was close to the length of a common double bond C=N indicating the iminium character of the cation. Quantum-chemical calculations [AM1, PM3, DFT/(PBE/3z), B3LYP/6-31G++(2d,p)] provided the geometry of cations similar to the experimental one. In the crystals under investigation motifs were observed of 0D, 1D, and 2D type mainly due to hydrogen bonds N-H···O and π-stacking interactions of the aromatic rings.
Similar content being viewed by others
References
Vorob’ev, A.Yu., Andreev, R.V., Borodkin, G.I., Gatilov, Yu.V., Shakirov, M.M., and Shubin, V.G., Abstracts of Papers, IX Nauchnaia shkola-konferentsiia po organicheskoi khimii (9th Conf. on Organic Chemistry). Moskva, 2006, p. 112.
Borodkin, G.I. and Shubin, V.G., Zh. Org. Khim., 2005, vol. 41, p. 487.
Sadykov, A.S., Kurbatov, Yu.V., and Zalyalieva, S.V., N-Iminy piridinovykh osnovanii (N-Imines of Pyridines Bases), Tashkent: FAN, 1982, p. 1.
Tamura, Y., Minamikawa, J., and Ikeda, M., Synthesis, 1977, p. 1.
Ivanov, A. Yu. and Lobanov, P.S., Sovremennye problemy organicheskoi khimii (Modern Problems of Organic Chemistry), St. Petersburg: Izd. SPb. Gos. Univ., 1998, vol. 12, p. 79.
Katritzky, A.R., Ballesteros, P., and Tomas, A.T., J. Chem. Soc., Perkin Trans. I, 1981, p. 1495.
Andreev, R.V. and Borodkin, G.I., Abstracts of Papers, Konf.. “Organicheskii sintez v novom stoletii” (Conf.: Organic Synthesis in New Century), St. Petersburg, 2002, p. 64.
Billert, T., Beckert, R., During, M., Wuckelt, J., Fehling, P., and Gцrls, H., J. Heterocycl. Chem., 2001, vol. 38, p. 205.
Abe, N., Odagiri, K., Otani, M., Fujinaga, E., Fujii, H., and Kakehi, A., J. Chem. Soc., Perkin Trans. I, 1999, p. 1339.
Matia, M.P., Garcнa-Navio, J.L., Vaquero, J.J., and Alvarez-Builla, J., Lieb. Ann., 1992, 1992, p. 777.
Palenik, G.J., Qian, K., Koziol, A.E., and Sisler, H.H., Inorg. Chem., 1990, vol. 29, p. 4016.
Filipenko, O.S., Aldoshin, S.M., Shilov, G.V., Makarova, N.I., Kharlanov, V.A., and Knyazhanskii, M.I., Izv. Akad. Nauk, Ser. Khim., 1995, p. 296.
Drexel, K.-P., Foro, S., Neunhoeffer, H., and Lindner, H.J., Z. Kristallogr., 1996, vol. 211, p. 665.
Lдmsд, M., Huuskonen, J., Rissanen, K., and Pursiainen, J., Chem. Eur. J., 1998, vol. 4, p. 84.
Bбtori, S., Hajys, G., Sбndor, P., and Messmer, A., J. Org. Chem., 1989, vol. 54, p. 3062.
Andreev, R.V., Borodkin, G.I., Gatilov, Yu.V., Shakirov, M.M., and Shubin, V.G., Zh. Org. Khim., 2004, vol. 40, p. 595.
Pozharskii, A.F., Kuz’menko, V.V., Foces-Foces, C., Llamas-Saiz, A.L., Claramunt, R.M., Sanz, D., and Elguero, J., J. Chem. Soc., Perkin Trans. II, 1994, p. 841.
Peters, K., Peters, E.-M., Irrgang, T., and Hetzheim, A., Z. Kristallogr. NCS., 1999, vol. 214, p. 167.
Koroleva, M.G., Dyablo, O.V., Pozharskii, A.F., and Starikova, Z.A., Khim. Geterotsikl. Soed., 2003, p. 1324.
Laus, G., Kahlenberg, V., Tцbbens, D.M., Jetti, R.K., Griesser, U.J., Schьtz, J., Kristeva, E., Wurst, K., and Schottenberger, H., Cryst. Growth Design., 2006, vol. 6, p. 404.
Kitaigorodskii, A.I., Zorkii, P.M., and Bel’skii, V.K., Stroenie organicheskogo veshchestva (Structure of Organic Substance), Moscow: Nauka, 1980, p. 396.
Enjalbert, R., Gleizes, A., and Trombe, J.-C., J. Mol. Struct., 1985, vol. 131, p. 1.
Chao, M., Schempp, E., and Rosenstein, R.D., Acta Crystllogr. B. Struct. Crystallogr. Chem., 1976, vol. 32, p. 288.
Gillespie, R.J., Molecular Geometry, New York: Reinhold, 1972.
Allen, F.H., Kennard, O., Watson, D.G., Brammer, L., Orpen, A.G., and Taylor, R., J. Chem. Soc., Perkin Trans. II, 1987, S1.
Gordon, A.J. and Ford, R.A., The Chemist’s Companion, New York: Wiley, 1972.
Aucott, S.M., Dale, S.H., Elsegood, M.R.J., Holmes, K.E., Gilby, L.M., and Kelly, P.F., Acta Cryst. C, 2005, vol. 61, o134.
Kuduva, S.S., Blaser, D., Boese, R., and Desiraju, G.R., J. Org. Chem., 2001, vol. 66, p. 1621.
Yang, X., Wu, D., Ranford, J.D., and Vittal, J.J., Crystal Growth Design, 2005, vol. 5, p. 41.
Coldatov, D.V. and Terekhova, I.S., Zh. Strukt. Khim., 2005, vol. 46, S5.
Rowland, R.S. and Taylor, R., J. Phys. Chem., 1996, vol. 100, p. 7384.
Sheldrick, G.M., Program for the Solution and Refinement of Crystal Structures, Gцttingen, 1997.
MOPAC Program Version 6.00, QCPE, no. 455.
Perdew, J.P., Burke, K., and Ernzerhof, M., Phys. Rev. Lett., 1996, vol. 77, p. 3865.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, p. 5648.
Stephens, P.J., Devlin, F.J., Chablowski, C.F., and Frisch, M.J., J. Phys. Chem., 1994, vol. 98, p. 11623.
Laikov, D.N., Chem. Phys. Lett., 1997, vol. 281, p. 151.
Laikov, D.N. and Ustynyuk, Yu.A., Izv. Akad. Nauk, Ser. Khim., 2005, p. 804.
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguyen, K.A., Su, S., Windus, T.L., Dupuis, M., and Montgomery, J.A., J. Comput. Chem., 1993, vol. 14, p. 1347.
Minkin, V.I., Simkin, B.Ya., and Minyaev, R.M., Kvantovaya khimiya organicheskikh soedinenii (Quantum Chemistry of Organic Compounds. Mechanisms of Reactions), Moscow: Khimiya, 1986, p. 10.
Metody polucheniya khimicheskikh reaktivov i preparatov (Methods of Chemicals Manufactury), Moscow: IPEA, 1966, vol. 14, p. 16.
Hall, S.A. and Spoerri, P.E., J. Am. Chem. Soc., 1940, vol. 62, p. 664.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © R.V. Andreev, G.I. Borodkin, A.Yu. Vorob’ev, Yu.V. Gatilov, V.G. Shubin, 2008, published in Zhurnal Organicheskoi Khimii, 2008, Vol. 44, No. 2, pp. 296–304.
For preliminary communication see [1].
Rights and permissions
About this article
Cite this article
Andreev, R.V., Borodkin, G.I., Vorob’ev, A.Y. et al. Molecular and crystal structure of 1-amino-X-pyrazinium mesitylenesulfonates. Russ J Org Chem 44, 292–301 (2008). https://doi.org/10.1134/S1070428008020188
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428008020188