Abstract
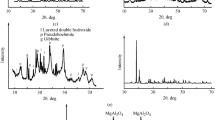
Barium aluminate BaAl2O4 of spinel structure was prepared by hydrothermal treatment at 150°С of a suspension of a gibbsite thermal activation product in an aqueous barium nitrate solution, followed by heat treatment of the precursors obtained. The product composition was studied by X-ray diffraction, thermal, microscopic, adsorption, and chemical analysis methods. Heat treatment of the hydrothermal reaction products at 850°С leads to the formation of single-phase barium aluminate BaAl2O4 with the specific surface area of ~50 m2 g–1 in the form of disk-shaped porous particles forming aggregates. The surface of BaAl2O4 particles is enriched in barium cations, which are relatively uniformly distributed over particles. The procedure allows reduction of the amount of the starting reactants and of the number of preparation steps, and also minimization or complete exclusion of the wash water formation.




Similar content being viewed by others
REFERENCES
Zhu, Z., Liu, F., and Zhang, W., Mater. Res. Bull., 2015, vol. 64, pp. 68–75. https://doi.org/10.1016/j.materresbull.2014.12.026
Li, S., Wang, W., Chen, Y., Zhang, L., Guo, J., and Gong, M., Catal. Commun., 2009, vol. 10, pp. 1048–1051. https://doi.org/10.1016/j.catcom.2008.12.064
Wako, A.H., Dejene, F.B., and Swart, H.C., J. Rare Earths, 2014, vol. 32, pp. 806–811. https://doi.org/10.1016/S1002-0721(14)60145-9
Maphiri, V.M., Mhlongo, M.R., Hlatshwayo, T.T., Motaung, T.E., Koao, L.F., and Motloung, S.V., Opt. Mater., 2020, vol. 109, ID 110244. https://doi.org/10.1016/j.optmat.2020.110244
Kim, D.H., Chin, Y.H., Kwak, J.H., Szanyi, J., and Peden, Ch.H.F., Catal. Lett., 2005, vol. 105, pp. 259–268. https://doi.org/10.1007/s10562-005-8700-y
Yadan, M. and Sharma, Y.C., Energy Conv. Manag., 2019, vol. 198, ID 111795. https://doi.org/10.1016/j.enconman.2019.111795
Mohapatra, A., Pattanaik, D.P., Anand, S., and Das, R.P., Ceram. Int., 2007, vol. 33, pp. 531–535. https://doi.org/10.1016/j.ceramint.2005.10.019
Rodehorst, U., Carpenter, M.A., Marion, S., and Henderson, C.M.B., Mineral. Mag., 2003, vol. 67, pp. 989–1013. https://doi.org/10.1180/0026461036750139
Chen, G.H. and Niu, D., J. Alloys Compd., 2006, vol. 413, pp. 319–322. https://doi.org/10.1016/j.jallcom.2005.07.001
Abakumov, A.M., Lebedev, O.I., Nistor, L., Tendeloo, G.V., and Amelinckx, S., Phase Trans., 2000, vol. 71, pp. 143–160. https://doi.org/10.1080/01411590008224545
Zhuzhgov, A.V., Kruglyakov, V.Yu., Suprun, E.A., Protsenko, R.S., and Isupova, L.A., Russ. J. Appl. Chem., 2021, vol. 94, no. 2, pp. 152–161. https://doi.org/10.1134/S107042722102004X
Bocanegra, S.A., Guerrero-Ruiz, А., Scelza, O.A., and de Miguel, С.Р., Catal. Ind., 2013, vol. 5, pp. 61–73. https://doi.org/10.1134/S2070050413010030
Belskaya, O.B., Stepanova, L.N., Gulyaeva, T.I., Golinskii, D.V., Belyi, A.S., and Likholobov, V.A., Kinet. Catal., 2015, vol. 56, no. 5, pp. 655–662. https://doi.org/10.1134/S0023158415050018
Patent RU 2264589, Publ. 2005.
Tanashev, Yu.Yu., Moroz, E.M., Isupova, L.A., Ivanova, A.S., Litvak, G.S., Amosov, Yu.I., Rudina, N.A., Shmakov, A.N., Stepanov, A.G., Kharina, I.V., Kul’ko, E.V., Danilevich, V.V., Balashov, V.A., Kruglyakov, V.Yu., Zolotarskii, I.A., and Parmon, V.N., Kinet. Catal., 2007, vol. 48, no. 1, pp. 153–161. https://doi.org/10.1134/S002315840701020X
Danilevich, V.V., Klimov, O.V., Nadeina, K.A., Gerasimov, E.Yu., Cherepanova, S.V., Vatutina, Yu.V., and Noskov, A.S., Superlat. Microstruct., 2018, vol. 120, pp. 148–160. https://doi.org/10.18412/1816-0387-2021-6-368-381
Danilevich, V.V., Isupova, L.A., and Parmon, V.N., Clean. Eng. Techn., 2021, vol. 3, ID 100118. https://doi.org/10.1016/j.clet.2021.100118
Buyanov, R.A. and Krivoruchko, O.P., Kinet. Katal., 1976, vol. 17, no. 3, pp. 765–775.
Ansaree, Md.J. and Upadhyay, S., Proc. Appl. Ceram., 2015, vol. 9, pp. 181–185. https://doi.org/10.2298/PAC1504181A
Funding
The study was performed within the framework of the government assignment for the Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences (grant no. 0239-2021-0004).
Author information
Authors and Affiliations
Contributions
A.V. Zhuzhgov and V.Yu. Kruglyakov: development of the experimental procedure and participation in the sample preparation, analysis of the results, and manuscript preparation; E.A. Suprun: electron microscopic examination of the samples; L.A. Isupova: significant contribution to the idea and design of the study, to the data interpretation, and to the final manuscript preparation.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 4, pp. 450–457, March, 2022 https://doi.org/10.31857/S0044461822040053
Rights and permissions
About this article
Cite this article
Zhuzhgov, A.V., Kruglyakov, V.Y., Suprun, E.A. et al. Synthesis of Barium Aluminate of Disk-Shaped Morphology Using the Product of Centrifugal Thermal Activation of Gibbsite. Russ J Appl Chem 95, 512–518 (2022). https://doi.org/10.1134/S1070427222040061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427222040061