Abstract
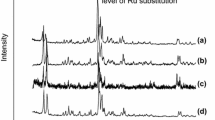
The properties of ZSM-12 zeolites prepared under hydrothermal conditions and microwave radiation influence were studied. The prepared zeolites are characterized by a wide range of physicochemical methods of analysis: X-ray diffraction analysis, low-temperature nitrogen adsorption/desorption, scanning electron microscopy, solid-state 27Al and 29Si NMR spectroscopy, infrared spectroscopy, temperature-programmed desorption of ammonia, infrared spectroscopy of adsorbed pyridine, and X-ray fluorescence elemental analysis. The calcined zeolites were impregnated with 0.5 wt % Pt, which performs the hydrogenation function in the reaction under study. The obtained materials were studied in the m-xylene isomerization reaction under the following conditions: Т = 300–440°С, WHSV = 1 h–1, Р(Н2) = 10 atm. It was found that, on the ZSM-12 MW catalyst, owing to its high acidity and fine particles, which promote high mass transfer, it is possible to increase the yields of m-xylene isomers, in particular p-xylene, to 36–65%.






Similar content being viewed by others
REFERENCES
Kuznetsov, P.S., Dementiev, K.I., Palankoev, T.A., Kalmykova, D.S., Malyavin, V.V., Sagaradze, A.D., and Maximov, A.L., Petrol. Chem., 2021, vol. 61, no. 6, pp. 649–662. https://doi.org/10.1134/s0965544121050182
Tsaplin, D.E., Naranov, E.R., Kulikov, L.A., Levin, I.S., Egazar’yants, S.V., Maximov, A.L., and Karakhanov, E.A., Petrol. Chem., 2021, vol. 61, pp. 815–824. https://doi.org/10.1134/s0965544121080089
Vorobkalo, V.A., Popov, A.G., Rodionova, L.I., Knyazeva, E.E., and Ivanova, I.I., Petrol. Chem., 2018, vol. 58, no. 12, pp. 1036–1044. https://doi.org/10.1134/s0965544118120137
Popov, A.G., Efimov, A.V., and Ivanova, I.I., Petrol. Chem., 2019, vol. 59, no. 7, pp. 691–694. https://doi.org/10.1134/S0965544119070168
Chen, D., Hu, X., Shi, L., Cui, Q., Wang, H., and Yao, H., Appl. Clay Sci., 2012, vol. 59–60, pp. 148–151. https://doi.org/10.1016/j.clay.2012.02.017
Onishchenko, M.I., Kulikov, A.B., and Maksimov, A.L., Petrol. Chem., 2017, vol. 57. N 14, pp. 1287–1294. https://doi.org/10.1134/S0965544117140079
Mingos, D.M.P., Advanced Mater., 1993, vol. 5, no. 11, pp. 857–859. https://doi.org/10.1002/adma.19930051115
Tsaplin, D.E., Makeeva, D.A., Kulikov, L.A., Maximov, A.L., and Karakhanov, E.A., Russ. J. Appl. Chem., 2018, vol. 91. N 12, pp. 1957–1962. https://doi.org/10.1134/S1070427218120066
Wang, Z., Sun, Q., Wang, D., Hong, Z., Qu, Z., and Li, X., Separation Purification Technol., 2019, vol. 209, pp. 1016–1026. https://doi.org/10.1016/j.seppur.2018.09.045
Košová, G. and Čejka, J., Collection Czech. Chem. Commun., 2002, vol. 67, no. 12, pp. 1760–1778. https://doi.org/10.1135/cccc20021760
Zhu, H.-B., Xia, Q.-H., Guo, X.-T., Su, K.-X., Hu, D., Ma, X., Zeng, D., and Deng, F., Mater. Lett., 2006, vol. 60, no. 17–18, pp. 2161–2166. https://doi.org/10.1016/j.matlet.2005.12.091
Wu, W., Wu, W., Kikhtyanin, O.V., Li, L., Toktarev, A.V., Ayupov, A.B., Khabibulin, J.F., Echevsky, G.V., and Huang, J., Appl. Catal. A: General, 2010, vol. 375, no. 2, pp. 279–288. https://doi.org/10.1016/j.apcata.2010.01.003
Parsafard, N., Peyrovi, M.H., and Rashidzadeh, M.N., Micropor. Mesopor. Mater., 2014, vol. 200, pp. 190–198. https://doi.org/10.1016/j.micromeso.2014.08.044
Sanhoob, M.A., Muraza, O., Yoshioka, M., Qamaruddin, M., and Yokop, T., J. Analyt. Appl. Pyrol., 2018, vol. 129, pp. 231–240. https://doi.org/10.1016/j.jaap.2017.11.007
Shavaleev, D.A., Pavlov, M.L., Dasimova, R.A., Sadovnikov, A.A., Sudin, V.V., Smirnova, E.M., Demikhova, N.R., Grigor’ev, Yu.V., and Maximov, A.L., Petrol. Chem., 2020, vol. 60, no. 9, pp. 1073–1079. https://doi.org/10.1134/S0965544120090182
Corma, A. and Sastre, E., J. Catal., 1991, vol. 129, no. 1, pp. 177–185. https://doi.org/10.1016/0021-9517(91)90021-U
Tsai, T.-C. and Wang, I., J. Catal., 1992, vol. 133, no. 1, pp. 136–145. https://doi.org/10.1016/0021-9517(92)90191-J
Glotov, A.P., Roldugina, E.A., Artemova, M.I., Smirnova, E.M., Demikhova, N.R., Stytsenko, V.D., Egazar’yants, S.V., Maximov, A.L., and Vinokurov, V.A., Russ. J. Appl. Chem., 2018, vol. 91, no. 8, pp. 1353–1362. https://doi.org/10.1134/s1070427218080141
Liu, Y., Zhou, X., Pang, X., Jin, Y., Meng, X., Zheng, X., Gao, X., and Xiao F.-Sh., ChemCatChem., 2013, vol. 5, no. 6, pp. 1517–1523. https://doi.org/10.1002/cctc.201200691
Glotov, A.P., Artemova, M.I., Demikhova, N.R., Smirnova, E.M., Ivanov, E.V., Gushchin, P.A., Egazar’yants, S.V., and Vinokurov, V.A., Petrol. Chem., 2019, vol. 59, no. 11, pp. 1226–1234. https://doi.org/10.1134/S0965544119110033
ACKNOWLEDGMENTS
The work was carried out using the equipment of the Center for Collective Use “Analytical Center for the Problems of Deep Refining of Oil and Petrochemistry” of the Institute of Petroleum Engineering, Russian Academy of Sciences.
Author information
Authors and Affiliations
Contributions
D.E. Tsaplin: synthesis of zeolites and catalysts based on them; V.A. Ostroumova: generalization of the results; L.A. Kulikov: catalytic experiments; E.R. Naranov: physicochemical analyzes by the methods of low-temperature adsorption–desorption of nitrogen, solid-state nuclear magnetic resonance spectroscopy on 27Al and 29Si nuclei; S.V. Egazar’yants: physicochemical analyzes by means of thermoprogrammed desorption of ammonia, infrared spectroscopy of adsorbed pyridine, and X-ray fluorescence spectroscopy; E.A. Karakhanov: formulation of the goals and objectives of the study for designing the synthesis of zeolite in microwave conditions and the selection of experimental conditions.
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest requiring disclosure in this article.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 9, pp. 1204–1213, January, 2021 https://doi.org/10.31857/S0044461821090103
Rights and permissions
About this article
Cite this article
Tsaplin, D.E., Ostroumova, V.A., Kulikov, L.A. et al. Comparison of Physicochemical Properties and Catalytic Activity in the m-Xylene Isomerization of Catalysts Based on ZSM-12 Zeolites Prepared at Hydrothermal Conditions and under the Action of Microwave Radiation. Russ J Appl Chem 94, 1292–1301 (2021). https://doi.org/10.1134/S1070427221090123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427221090123