Abstract
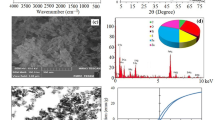
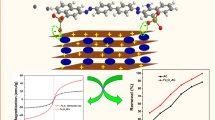
Magnetic nanoparticles and clay minerals combine to form a class of advanced nanocomposites that would possess exceptional adsorption, magnetism, and stability. In this work, an environmentally friendly nanocomposite was successfully fabricated by functionalizing natural clay. Bentonite сlay/meghemite nanocomposite was synthesized by the co-precipitation method and used to Methyl Orange pollutant removal as a toxic anionic dye from aqueous solutions. Physical and structural characteristics of the synthesized adsorbent were assessed using different techniques including Fourier transform infrared spectrometer, scanning electron microscopy, vibrating sample magnetometry, and X-ray diffraction. The saturation magnetization of maghemite and bentonite/maghemite nanocomposite are 50.9 and 28.5 emu g–1, respectively. The average size of the synthesized maghemite nanoparticles calculated by the Scherer equation was 16.60 nm. Different kinetic and thermodynamic models and isotherms of the adsorption process were also investigated. The adsorption capacity became equilibrium after 120 min. The consistency of the adsorption process with the pseudo-second-order kinetic model was confirmed by studying its kinetic data. Investigating the equilibrium isotherm data at different temperatures showed better compatibility with the Freundlich model. The negative values of ΔG and positive values of ΔH obtained from adsorption thermodynamic study revealed that Methyl Orange adsorption from aqueous samples is spontaneous and endothermic. The optimal parameters for Methyl Orange removal by synthesized adsorbent were determined by MINITAB 17 under response surface methodology (RSM). The maximum adsorption capacity of dye adsorption of 56.79 mg g–1 was obtained under optimum conditions of pH = 4, adsorbent dose of 1 g L–1 and dye concentration of 90 mg L–1.












Similar content being viewed by others
REFERENCES
Gupta, V.K. and Saleh, T.A., Env. Sci. & Pollution Res., 2013, vol. 20, no. 5, pp. 2828–2843. https://doi.org/10.1007/S11356-013-1524-1
Paul, J., et al., Appl. Radiat. & Isot., 2011, vol. 69, no. 7, pp. 982–987. https://doi.org/10.1016/j.apradiso.2011.03.009
Javanbakht, V., Alavi, S.A., and Zilouei, H., Water Sci. & Technol., 2014, vol. 69, no. 9, p. 1775. https://doi.org/10.2166/wst.2013.718
Javanbakht, V. and Ghoreishi, S.M., Adsorption Science & Technology, 2017, vol. 35, nos. 1–2, pp. 241–260. https://doi.org/10.1177/0263617416674474
Sabouri, M.R., Et Al., Process Safety and Environmental Protection, 2019, vol. 126, pp. 182–192. https://doi.org/10.1016/j.psep.2019.04.006
Saravanan, R., et al., Colloid & Interface Science, 2015, vol. 452, pp. 126-133. https://doi.org/10.1016/j.jcis.2015.04.035
Javanbakht, V., et al., Powder Technology, 2016, vol. 302, pp. 372-383. https://doi.org/10.1016/j.powtec.2016.08.069
Al-Kdasi, A., et al., Global Nest, The Int. J, 2004, vol. 6, no. 3, pp. 222–230.
Chen, S., et al., Desalination, 2010, vol. 2521–3, pp. 149–156. https://doi.org/10.1016/j.desal.2009.10.010
Ofomaja, A.E. and Ho, Y.-S., Bioresource Technology, 2008, vol. 99, no. 13, pp. 5411–5417. https://doi.org/10.1016/j.biortech.2007.11.018
Royer, B., et al., Hazardous Materials, 2009, vol. 164, nos. 2–3, pp. 1213–1222. https://doi.org/10.1016/j.jhazmat.2008.09.028
Brookstein, D.S., Dermatologic Clinics, 2009, vol. 27, no. 3, pp. 309–322. https://doi.org/10.1016/j.det.2009.05.001
Carneiro, P.A., et al., Hazardous Materials, 2010, vol. 174, nos. 1–3, pp. 694–699. https://doi.org/10.1016/j.jhazmat.2009.09.106
Mehrabi, M. and Javanbakht, V, Materials Science, Materials In Electronics, 2018, vol. 29, no. 12, pp. 9908–9919. https://doi.org/10.1007/S10854-018-9033-0
Bayat, M., et al., International Journal of Biological Macromolecules, 2018, vol. 116, pp. 607–619. https://doi.org/10.1016/J.Ijbiomac.2018.05.012
Erfani, M. and Javanbakht, V., International Journal of Biological Macromolecules, 2018, vol. 114, pp. 244–255. https://doi.org/10.1016/J.Ijbiomac.2018.03.003
Mirzaei, S. and Javanbakht, V., International Journal of Biological Macromolecules, 2019, vol. 134, pp. 1187–1204. https://doi.org/10.1016/j.ijbiomac.2019.05.119
Haque, E., et al., Hazardous Materials, 2011, vol. 185, no. 1, pp. 507–511. https://doi.org/10.1016/j.jhazmat.2010.09.035
Vaez, Z. and Javanbakht, V., Photochemistry & Photobiology A, Chemistry, 2019, vol. 388, p. 112064. https://doi.org/10.1016/j.jphotochem.2019.112064
Chen, Z.-X., et al., Colloid and Interface Science, 2011, vol. 363, no. 2, pp. 601–607. https://doi.org/10.1016/j.jcis.2011.07.057
Azizian, S., et al., Chemical Engineering, 2009, vol. 146, no. 1, pp. 36–41. https://doi.org/10.1016/j.cej.2008.05.024
Rahpeima, S., et al., Inorganic and Organometallic Polymers and Materials, 2018, vol. 28, no. 1, pp. 195–211. https://doi.org/10.1007/S10904-017-0688-4
Demirbas, E., et al., Bioresource Technology, 2008, vol. 99, no. 13, pp. 5368–5373. https://doi.org/10.1016/j.biortech.2007.11.019
Keyvani, F., et al., Solid State Sciences, 2018, Vol. 83, pp. 31–42. https://doi.org/10.1016/j.solidstatesciences.2018.06.007
Alver, E. and Metin, A.Ü. Chemical Engineering, 2012, vol. 200, pp. 59–67. https://doi.org/10.1016/j.cej.2012.06.038
Wang, X.S., et al., Hazardous Materials, 2008. Vol. 157, nos. 2–3, pp. 374–385. https://doi.org/10.1016/j.jhazmat.2008.01.004
Rosa, S., et al., Hazardous Materials, 2008, vol. 155, nos. 1–2, pp. 253–260. https://doi.org/10.1016/j.jhazmat.2007.11.059
Tanabtabzadeh, M.S., et al., Waste and Biomass Valorization, 2019, vol. 10, no. 3, pp. 641–653. https://doi.org/10.1007/S12649-017-0086-8
Liu, Y., et al., Chemical Engineering, 2013, vol. 218, pp. 46–54. https://doi.org/10.1016/j.cej.2012.12.027
Javanbakht, V., et al., Protection of Metals and Physical Chemistry of Surfaces, 2017, vol. 53, no. 4, pp. 693–702. https://doi.org/10.1134/S2070205117040086
Javanbakht, V., et al., Chemical and Pharmaceutical Research, 2016, vol. 8, no. 4, pp. 846–852.
Demirbas, A., Hazardous Materials, 2009, vol. 167, no. 1, pp. 1–9. https://doi.org/10.1016/j.jhazmat.2008.12.114
Mallakpour, S. and Hatami, M., Designed Monomers and Polymers, 2011, vol. 14, no. 5, pp. 461–473. https://doi.org/10.1163/138577211X587654
Oliveira, L.C., et al., Carbon, 2002, vol. 40, no. 12, pp. 2177–2183. https://doi.org/10.1016/S0008-62230200076-3
Sareban, Z. and Javanbakht, V., Korean Journal of Chemical Engineering, 2017, vol. 34, no. 11, pp. 2886–2900. https://doi.org/10.1007/S11814-017-0216-9
Aeenjan, F. and Javanbakht, V., Research On Chemical Intermediates, 2018, vol. 44, no. 3, pp. 1459–1483. https://doi.org/10.1007/S11164-017-3179-X
Gnanaprakash, G., et al., Materials Chemistry and Physics, 2007, vol. 103, no. 1, pp. 168–175. https://doi.org/10.1016/j.matchemphys.2007.02.011
Lu, A.H., et al., Angewandte Chemie International Edition, 2007, vol. 46, no. 8, pp. 1222–1244. https://doi.org/10.1002/anie.200602866
Chen, L., et al., Applied Clay Science, 2016, vol. 127, pp. 143–163. https://doi.org/10.1016/j.clay.2016.04.009
Rechendorff, K., et al., Langmuir, 2006, vol. 22, no. 26, pp. 10885–10888. https://doi.org/10.1021/la0621923
Özcan, A.S. and Özcan, A., Colloid and Interface Science, 2004, vol. 276, no. 1, pp. 39–46. https://doi.org/10.1016/j.jcis.2004.03.043
Darezereshki, E., et al., Materials Science In Semiconductor Processing, 2013, vol. 16, no. 1, pp. 221–225. https://doi.org/10.1016/j.mssp.2012.08.007
Tan, Y., et al., Chemical Engineering, 2012, vol. 191, pp. 104–111. https://doi.org/10.1016/j.cej.2012.02.075
Xie, M., et al., Alloys and Compounds, 2015, vol. 647, pp. 892–905. https://doi.org/10.1016/j.jallcom.2015.06.065
Zhao, G., et al., The Open Colloid Science, 2010, vol. 4, p. 1. https://doi.org/10.2174/1876530001104010019
Foo, K. and Hameed, B., Chemical Engineering, 2010, vol. 156, no. 1, pp. 2–10. https://doi.org/10.1016/j.cej.2009.09.013
Gautam, R.K., et al., Molecular Liquids, 2015, vol. 204, pp. 60–69. https://doi.org/10.1016/J.Molliq.2015.01.038
Bayramoğlu, G. and Arica, M.Y., Chemical Engineering, 2008, vol. 139, no. 1, pp. 20–28. https://doi.org/10.1016/j.cej.2007.07.068
Ma, J., et al., ACS Applied Materials & Interfaces, 2012, vol. 4, no. 11, pp. 5749–5760. https://doi.org/10.1021/am301053m
Hao, Y.-M., et al., Hazardous Materials, 2010, vol. 184, no. 1, pp. 392–399. https://doi.org/10.1016/j.jhazmat.2010.08.048
Chang, Y.C. and Chen, D.H., Macromolecular Bioscience, 2005, vol. 5, no. 3, pp. 254–261. https://doi.org/10.1002/Mabi.200400153
Aksu, Z. and Gönen, F., Separation & Purification Technology, 2006, vol. 49, no. 3, pp. 205–216. https://doi.org/10.1016/j.seppur.2005.09.014
Can, M.Y., et al., Bioresource Technology, 2006, vol. 97, no. 14, pp. 1761–1765. https://doi.org/10.1016/j.biortech.2005.07.017
Ghorbani, F., et al., Chemical Engineering, 2008, vol. 145, no. 2, pp. 267–275. https://doi.org/10.1016/j.cej.2008.04.028
Ahmed, M., et al., Materials Science & Engineering, B, 2013, vol. 178, no. 10, pp. 744–751. https://doi.org/10.1016/j.mseb.2013.03.011
Dinu, M.V. and Dragan, E.S., Chemical Engineering, 2010, vol. 160, no. 1, pp. 157–163. https://doi.org/10.1016/j.cej.2010.03.029
Wang, L. and Wang, A., Bioresource Technology, 2008, vol. 99, no. 5, pp. 1403–1408. https://doi.org/10.1016/j.biortech.2007.01.063
Zhu, H., et al., Applied Surface Science, 2011, vol. 258, no. 4, pp. 1337–1344. https://doi.org/10.1016/j.apsusc.2011.09.045
Kamaru, A.A., et al., Desalination & Water Treatment, 2016, vol. 57, no. 40, pp. 18836–18850. https://doi.org/10.1080/19443994.2015.1095122
Shariati-Rad, M., et al., International Nano Letters, 2014, vol. 4, no. 4, pp. 91–101. https://doi.org/10.1007/S40089-014-0124-5
Fan, J., et al., Colloid and Interface Science, 2016, vol. 470, pp. 229–236. https://doi.org/10.1016/j.jcis.2016.02.045
Yang, H.-C., et al., Colloid and Interface Science, 2017, vol. 505, pp. 67–78. https://doi.org/10.1016/j.jcis.2017.05.075
Karthika, J. and Vishalakshi, B., International Journal of Biological Macromolecules, 2015, vol. 81, pp. 648–655. https://doi.org/10.1016/j.ijbiomac.2015.08.064
ACKNOWLEDGMENT
Financial support of this work by ACECR Institute of Higher Education (Isfahan Branch) is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rezaei, S., Rahpeima, S., Esmaili, J. et al. Optimization by Response Surface Methodology of the Adsorption of Anionic Dye on Superparamagnetic Clay/Maghemite Nanocomposite. Russ J Appl Chem 94, 533–548 (2021). https://doi.org/10.1134/S1070427221040145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427221040145