Abstract
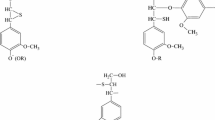
Gas chromatography-mass spectrometry was used to study the composition of the degradation products of ionic liquids and wood released into the vapor phase during the dissolution of lignocellulosic biomass in 1-butyl-3-methylimidazolium acetate and methyl sulfate and their binary mixtures with dimethyl sulfoxide. It was found that the volatile compounds form at temperatures >100°C, and at 150°C their content can reach 6% of the mass of the initial reaction mixture. They are dominated by methanol and butanol, esters of acetic and benzoic acids, butyl and methyl imidazole, aromatic and aliphatic amines, and toluene. The presence of wood has a significant effect on the thermal decomposition of ionic liquids, including due to the presence of residual moisture. The use of the 1-butyl-3-methylimidazolium methyl sulfate – dimethyl sulfoxide system as a wood solvent leads to the release of many toxic and foul-smelling organosulfur compounds into the vapor phase.

Similar content being viewed by others
REFERENCES
Kilpeläinen, I., Xie, H., King, A., Granstrom, M., Heikkinen, S., and Argyropoulos, D.S., J. Agric. Food. Chem., 2007, vol. 55, no. 22, pp. 9142–9148. https://doi.org/10.1021/jf071692e.
Lan, W., Liu, C.-F., and Sun, R.-C., J. Agric. Food. Chem., 2011, vol. 59, no. 16, pp. 8691–8701. https://doi.org/10.1021/jf201508g
Ladesov, A.V., Kosyakov, D.S., Bogolitsyn, K.G., and Gorbova, N.S., Russ. J. Phys. Chem., A, 2015, vol. 89, no. 10, pp. 1814–1820. https://doi.org/10.1134/S0036024415100167
Made, M., Liu, J.-F., and Pang, L., Environ. Sci. Technol., 2015, vol. 49, no. 21, pp. 12611–12627. https://doi.org/10.1021/acs.est.5b03123
Shill, K., Padmanabhan, S., Xin, Q., Prausnitz, J.M., Clark, D.S., and Blanch, H.W., Biotechnol. Bioeng., 2011, vol. 108, no. 3, pp. 511–520. https://doi.org/10.1002/bit.23014
.Ladesov, A.V., Belesov, A.V., Kuznetsova, M.V., Pochtovalova, A.S., Malkov, A.V., Shestakov, S.L., and Kosyakov, D.S., Russ. J. Appl. Chem., 2018, vol. 91, no. 4, pp. 663–670. https://doi.org/10.1134/S1070427218040201
.Pinkert, A., Goeke, D.F., Marsh, K.N., and Pang, S., Green Chem., 2011, vol. 13, no. 11, pp. 3124. https://doi.org/10.1039/c1gc15671c
Pu, Y., Jiang, N., and Ragauskas, A.J., J. Wood Chem. Technol., 2007, vol. 27, no. 1, pp. 23–33. https://doi.org/10.1080/02773810701282330
Ovejero-Pérez, A., Rigual, V., Domínguez, J.C., Alonso, M.V., Oliet, M., and Rodriguez, F., Int. J. Biol. Macromol., 2020, vol. 157, no. 15, pp. 461–469. https://doi.org/10.1016/j.ijbiomac.2020.04.194
Brandt, A., Ray, M.J., To, T.Q., Leak, D.J., Murphy, R.J., and Welton, T., Green Chem., 2011, vol. 13, no. 9, pp. 2489–2499. https://doi.org/10.1039/c1gc15374a
Zhang, P., Dong, S.-J., Ma, H.-H., Zhang, B.-X., Wang, Y.-F., and Hu, X.-M., Ind. Crops Prod., 2015, vol. 76, no. 1, pp. 688–696. https://doi.org/10.1016/j.indcrop.2015.07.037
Castro, M.C., Rodríguez, H., Arce, A., and Soto, A.Ind. Eng. Chem. Res., 2014, vol. 53, no. 29, pp. 11850–11861. https://doi.org/10.1021/ie501956x
Verdía, P., Brandt, A., Hallett, J.P., Ray, M.J., and Welton, T., Green Chem., 2014, vol. 16, no. 3, p. 1617. https://doi.org/10.1039/c3gc41742e
Clough, M.T., Geyer, K., Hunt, P.A., Mertes, J., and Welton, T., Phys. Chem. Chem. Phys., 2013, vol. 15, no. 47, pp. 20480–20495. https://doi.org/10.1039/c3cp53648c
Belesov, A.V., Ladesov, A.V., Pikovskoi, I.I., Faleva, A.V., and Kosyakov, D.S.,Molecules, 2020, vol. 25, no. 11, p. 2479. https://doi.org/10.3390/molecules25112479
Chiarotto, I., Feroci, M., and Inesi, A., New J. Chem., 2017, vol. 41, no. 16, pp. 7840–7843. https://doi.org/10.1039/c7nj00779e
Sowmiah, S., Srinivasadesikan, V., Tseng, M.C., and Chu, Y.H., Molecules, 2009, vol. 14, no. 9, pp. 3780–3813. https://doi.org/10.3390/molecules14093780
Yan, F., Dhumal, N.R., and Kim, H.J., Phys. Chem. Chem. Phys., 2017, vol. 19, no. 2, pp. 1361–1368. https://doi.org/10.1039/c6cp06556b
Wendler, F., Todi, L.N., and Meister, F., Thermochim. Acta, 2012, vol. 528, no. 1, pp. 76–84. https://doi.org/10.1016/j.tca.2011.11.015
Ohtani, H., Ishimura, S., and Kumai, M., Anal. Sci., 2008, vol. 24, no. 10, pp. 1335–1340. https://doi.org/10.2116/analsci.24.1335
ACKNOWLEDGMENTS
The study was performed using the equipment of the Core Facility Center “Arktika” of the Northern (Arctic) Federal University.
Funding
The study was financially supported by the Russian Science Foundation (project 18-73-00282). The Belesov’s work was supported by a grant from the Russian Foundation for Basic Research for postgraduate students (project 20-33-90153-asp).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 3, pp. 353–362, January, 2021 https://doi.org/10.31857/S0044461821030105
Rights and permissions
About this article
Cite this article
Belesov, A.V., Ladesov, A.V., Pokryshkin, S.A. et al. Study of the Composition of Volatile By-Products, Formed by Dissolution of Wood in Ionic Liquids Based on 1-Butyl-3-Methylimidazolium. Russ J Appl Chem 94, 337–346 (2021). https://doi.org/10.1134/S1070427221030101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427221030101