Abstract
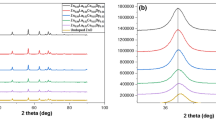
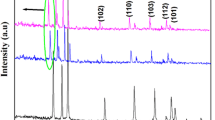
Zinc oxide nanoparticles were co-doped with varying concentrations of lanthanum (La) and cerium (Ce) ions using co-precipitation method. The resulting powders were calcined in a muffle furnace for 1 h at a temperature of 500°C to produce La,Ce-doped ZnO nanoparticles of varying stoichiometry viz. (Zn0.98La0.01Ce0.01O, Zn0.96La0.02Ce0.02O, Zn0.94La0.03Ce0.03O, and Zn0.92La0.04Ce0.04O). This method of co-doping is cost effective and does not require any complex procedure, equipment or inert gases. The synthesized samples were characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM) to study the size, phases and grain morphology. XRD patterns revealed the hexagonal wurtzite phase of the synthesized samples. The average size of the undoped and La,Ce co-doped ZnO nanoparticles, as calculated from XRD pattern, was found to be 25 nm. Further, the size of co-doped nanoparticles decreased significantly with increasing dopant concentration. Optical properties were studied through UV-Visible spectrophotometry. The photocatalytic activities of undoped and La,Ce co-doped ZnO nanoparticles were examined by observing the decomposition of Rhodamine B dye under UV lamp within 0–80 min. The Rhodamine B (RB) dye solution was efficiently photo-degraded within 35 min when using Zn0.92La0.04Ce0.04O as catalyst, mechanism of which has been thoroughly discussed below. Further, photoluminescence (PL) studies revealed the narrowing of band gap with increase in concentration of dopant ions as indicated by the red shift in the PL emission spectrum of co-doped nanoparticles.








Similar content being viewed by others
REFERENCES
Xu, H., Wang, W., and Zhu, W., J. Phys. Chem., 2006, vol. 110, pp. 13829–13834. https://doi.org/10.1021/jp061934y
El-Sayed, M.A., Acc. Chem. Res., 2001, vol. 34, pp. 257–264. https://doi.org/10.1021/ar960016n
Abdullah, M., Morimoto, T., and Okuyama, K., Adv. Funct.Mater., 2003, vol. 13, pp. 800–804. https://doi.org/10.1002/adfm.200304330
Karnan, T., Arul, S., and Kumar, S.S., J. Mol. Struct., 2016, vol. 1125, pp. 358–365. https://doi.org/10.1016/j.molstruc.2016.07.029
VanDijken, A., Meulenkamp, E.A., Vanmaekelbergh, D., and Meijerink, A., J., Luminesc., 2000, vol. 90, pp. 123–128. https://doi.org/10.1016/S0022-2313(99)00599-2
Volbers, N., Zhou, H., Knies, C., Pfisterer, D., Sann, J., Hofmann, D.M., and Meyer, B.K., Appl. Phys. A, 2007, vol. 88, pp. 153–155. https://doi.org/10.1007/s00339-007-3960-6
Rau, U., and Schmidt, M., Thin Solid Films, 2001, vol. 387, pp. 141–146. https://doi.org/10.1016/S0040-6090(00)01737-5
Soki, T., Hatanaka, Y., and Look, D., Appl. Phys. Lett., 2003, vol. 76, pp. 3257–3259. https://doi.org/10.1063/1.126599
Tang, Z., Wong, G., Yu, P., Kawasaki, M., Ohtomo, A., and Koinuma, H., Appl. Phys. Lett., 1998, vol. 72, pp. 3270–3277. https://doi.org/10.1063/1.121620
Dindar, B. and Icli, S. J. Photochem. Photobiol. A, 2001, vol. 140, pp. 263–268. https://doi.org/10.1016/S1010-6030(01)00414-2
Hoffmann, M.R., Martin, S.T., Choi, W.Y., and Bahnemann, D.W., Chem. Rev., 1995, vol. 95, pp. 69–96. https://doi.org/10.1021/cr00033a004
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K., and Taga, Y., Science, 2001, vol. 293, pp. 269–271. https://doi.org/10.1126/science.1061051
Qiu, R.L., Zhang, D.D., Yueqi, M., Lin, S., Brewer, E., Huang, X.F., et al., J. Hazard.Mater., 2008, vol. 156, pp. 80–85. https://doi.org/10.1016/j.jhazmat.2007.11.114
Kumaran, N.N. and Muraleedharan, K., J.Water.Proc.Eng., 2017, vol. 17, pp. 264–270. https://doi.org/10.1016/j.jwpe.2017.04.014
Nair, M., Luo, Z.H., and Heller, A., Ind. Eng. Chem. Res., 1993, vol. 32, pp. 2318–2323. https://doi.org/10.1021/ie00022a015
Boer, K.W., Survey of Semiconductor Physics, Van Nostrand Reinhold: New York, 1990, pp. 249.
Linsebigler, A.L., Lu, G.Q., and Yates, Jr.J.T., Chem. Rev., 1995, vol. 95, pp. 735–758. https://doi.org/10.1021/cr00035a013
Romero, M., Blanco, J., Sanchez, B., Vidal, A., Malato, S., Cardona, A., et. al., Sol. Energy, 1999, vol. 66, pp. 169–182. https://doi.org/10.1016/S0038-092X(98)00120-0
Guan, K.S. and Yin, Y.S., Mater. Chem. Phys., 2015, vol. 92, no. 19, pp. 16905–16912. https://doi.org/10.1021/acs.jpcc.5b02485
Iqbal, J., Liu, X., Zhu, H., Wua, Z.B., Zhang, Y., Yu, D., and Yu, R., Acta Mater., 2009, vol. 57, pp. 4790–4796. https://doi.org/10.1016/j.actamat.2009.06.056
Pascariu, P., Homocianu, M., Cojocaru, C., Samoila, P., Airinei, A., and Suchea, M., Appl. Surf. Sci., 2019, vol. 476, pp. 16–27. https://doi.org/10.1016/j.apsusc.2019.01.077
Romero, M., Blanco, J., Sanchez, B., Vidal, A., Malato, S., Cardona, A.I., et al., Sol. Energy, 1999, vol. 66, pp. 169–182. https://doi.org/10.1016/S0038-092X(98)00120-0
Guan, K.S. and Yin, Y.S., Mater Chem. Phys., 2005, vol. 92, pp. 10–15. https://doi.org/10.1016/j.matchemphys.2004.01.044
Wanger, C.D., Riggs, W.M., Davis, L.E., and Moulder, F.J., Handbook of XPS, Eden Prairie MN: Perkin Elmer Corporation., 1979. https://doi.org/10.1002/sia.740030412
Arguello, C.A., Rousseau, D.L., and Porto, S.P.S., Phys. Rev., 1969, vol. 181, pp. 1351–1363. https://doi.org/10.1103/PhysRev.181.1351
Zhang, W.F., Zhang, M.S., Yin, Z., and Chen, Q., Appl. Phys. B, 2000, vol. 70, pp. 261–265. https://doi.org/10.1007/s003400050043
Khatamian, M., Khandar, A.A., Divband, B., Haghighi, M., and Ebrahimiasl, S., J. Mol. Catal. A. Chem., 2012, vol. 365, pp. 120–127. https://doi.org/10.1016/j.molcata.2012.08.018
Sin, J.C., Lam, S.M., Lee, K.T., and Mohamed, A.R., J. Colloid Interf. Sci., 2013, vol. 401, pp. 40–49. https://doi.org/10.1016/j.jcis.2013.03.043
Rahman, A. and Jayaganthan, R., Trans. Indian Inst. Met., 2016, vol. 70, pp. 1063–1074. https://doi.org/10.1007/s12666-016-0897-5
Li, X.Z., Li, F.B., Yang, C.L., and Ge, W.K., J. Photochem. Photobiol. A, 2001, vol. 141, pp. 209–217. https://doi.org/10.1016/S1010-6030(01)00446-4
Jing, L.Q., Sun, X.J., Cai, W.M., Xu, Z.L., Du, Y.G., and Fu, H.G., J. Phys. Chem. Solid., 2003, vol. 64, pp. 615–623. https://doi.org/10.1016/S0022-3697(02)00362-1
Yamashita, H., Ichihashi, Y., Zhang, S.G., Matsumurab, Y., Soumab, Y., Tatsumic, T., et.al., Appl. Surf. Sci.,1997, vol. 121, pp. 305–311. https://doi.org/10.1016/S0169-4332(97)00311-5
Zou, D., Yan, D., Xiao, L., and Dong, Y., Surf. Coat. Technol., 2008, vol. 202, pp. 1928–1934. https://doi.org/10.1016/j.surfcoat.2007.08.022
Elilarassi, R. and Chandrasekaran, G., J. Mater. Sci. Mater. Electron, 2010, vol. 21, pp. 1168–1173. https://doi.org/10.1007/s10854-009-0041-y
Yayapao, O., Thongtem, T., Phuruangrat, A., and Thongtem, S., J. Alloy. Compnd., 2013, vol. 576, pp. 72–79. https://doi.org/10.1016/j.jallcom.2013.04.133
Anbuvannan, M., Ramesh, M., Viruthagiri, S.N., and Kannadasan, N., Mater. Sci. Semicond. Process,2015, vol. 39, pp. 621–628. https://doi.org/10.1016/j.mssp.2015.06.005
Zhang, W.F., Zhang, M.S., Yin, Z., and Chen, Q., Appl. Phys., 2000, vol. 70, pp. 261–265. https://doi.org/10.1007/s003400050043
Zeng, J.H., Yu, Y.L., Wang, Y.F., and Lou, T., J. Acta.Mater., 2009, vol. 57, pp. 1813–1820. https://doi.org/10.1016/j.actamat.2008.12.021
Iqbal, J., Wang, B., Liu, X.F., Yu, D.P., He, B., and Yu, R.H., New. J. Phys., 2009, vol. 11, pp. 63009–63014. https://doi.org/10.1088/1367-2630/11/6/063009
Majid, A. and Ali, A., J. Phys. D. Appl. Phys., 2009, vol. 42, pp. 45412–45416. https://doi.org/10.1088/0022-3727/42/4/045412
Bhatia, S. and Verma, N., Mater. Res. Bull, 2017, vol. 95, pp. 468–476. https://doi.org/10.1016/j.materresbull.2017.08.019
Berggren, K.F. and Sernelius, B.E., Phys. Rev., 1971, vol. 24, pp. 1971–1986. https://doi.org/10.1103/PhysRevB.24.1971
George, A., Sharma, S.K., Chawla, S., Malik, M.M., and Qureshi, M.S., J. Alloys Compd., 2011, vol. 509, pp. 5942–5946. https://doi.org/10.1016/j.jallcom.2011.03.017
Wang, S., Bai, L., and Ao, X., RSC Adv., 2018, vol. 8, pp. 36745–36753. https://doi.org/10.1039/C8RA06778C
Chang, C.J., Lin, C.Y., and Hsu, M.H., J. Taiwan. Inst.Chem. Eng., 2014, vol. 45, pp. 1954–1963. https://doi.org/10.1016/j.jtice.2014.03.008
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Irtiqa, S., Rahman, A. Photocatalytic Studies of La,Ce Co-Doped ZnO Nanoparticles. Russ J Appl Chem 93, 1906–1919 (2020). https://doi.org/10.1134/S1070427220120137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220120137