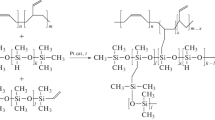
Abstract
Synthesis of monomers is the key and most labor-consuming step in the development of highly selective membrane materials. Polyalkylenesiloxanes show promise for separation of vapors of organic components from gas mixtures. The paper considers two approaches to the synthesis of 1,1,3,3,5,5-hexamethyl-2-oxa-1,3,5-trisilacyclohexane, a monomer for preparing poly-bis(dimethylsilmethylene)dimethylsiloxane, a promising polymer material for gas-separation and pervaporation membranes. Modified synthesis procedures using both approaches, closure of the six-membered ring via formation of the Si–O–Si or Si–C bond, are suggested. Comparative analysis shows that, among organomagnesium cyclization methods, the one-step method in a diethyl ether or diethyl glycol dibutyl ether should be preferred. The suggested procedure allows reaching the monomer yield as high as 75–80% and more.




Similar content being viewed by others
REFERENCES
Apel, P.Yu., Bobreshova, O.V., Volkov, A.V., Volkov, V.V., Nikonenko, V.V., Stenina, I.A., Filippov, A.N., Yampolskii, Yu.P., and Yaroslavtsev, A.B., Membr. Membr. Technol., 2019, vol. 1, no. 2, pp. 45–63. https://doi.org/10.1134/S2517751619020021
Akhmetshina, A.I., Yanbikov, N.R., Atlaskin, A.A., Trubyanov, M.M., Mechergui, A., Otvagina, K.V., Razov, E.N., Mochalova, A.E., and Vorotyntsev, I.V., Membranes, 2019, vol. 9, no. 1, p. 9. https://doi.org/10.3390/membranes9010009
Borisov, I., Bakhtin, D., Luque-Alled, J.M., Rybakova, A., Makarova, V., Foster, A.B., Harrison, W.J., Volkov, V., Polevaya, V., Gorgojo, P., Prestat, E.M., Budd, P., and Volkov, A., J. Mater. Chem. A, 2019, vol. 7, pp. 6417–6430. https://doi.org/10.1039/C8TA10691F
Storozhuk, I.P., Pavlukovich, N.G., Korobkina, A.V., and Kagramanov, G.G., Membr. Membr. Technol., 2020, vol. 2, pp. 71–75. https://doi.org/10.1134/S2517751620020043
Rasmussen, S.B., Huang, J., Riisager, A., Hamm, H., Rogez, J., Winnick, J., Wassserscheid, P., and Fehrmann, R., ECS Trans., 2007, vol. 3, no. 35, pp. 49–59. https://doi.org/10.1149/1.2798646
Lytkina, A.A., Orekhova, N.V., Ermilova, M.M., Yaroslavtsev, A.B., Petriev, I.S., and Baryshev, M.G., Petrol. Chem., 2017, vol. 57, no. 13, pp. 1219–1227. https://doi.org/10.1134/S0965544117130072
Aydin, S., Yesil, H., and Tugtas, A.E., Bioresource Technol., 2018, vol. 250, pp. 548–555. https://doi.org/10.1016/j.biortech.2017.11.061
Grushevenko, E.A., Podtynnikov, I.A., and Borisov, I.L., Russ. J. Appl. Chem., 2019, vol. 92, no. 11, pp. 1593–1601. https://doi.org/10.1134/S1070427219110168
Smirnova, N.N., Russ. J. Appl. Chem., 2019, vol. 92, no. 2, pp. 222–227. https://doi.org/10.1134/S1070427219020083
Finkelshtein, E.S., Ushakov, N.V., and Gringolts, M.L., Polycarbosilanes based on silicon–carbon cyclic monomers, Silicon Polymers, Berlin: Springer, 2010, pp. 111–159. https://doi.org/10.1007/12_2009_39
Borisov, I.L., Ushakov, N.V., Volkov, V.V., and Finkel’shtein, E.Sh., Petrol. Chem., 2016, vol. 56, no. 9, pp. 798–804. https://doi.org/10.1134/S0965544116090024
Stern, S.A., Shah, V.M., and Hardy, B.J., J. Polym. Sci. Phys., 1987, vol. 25, no. 6, pp. 1263–1298. https://doi.org/10.1002/polb.1987.090250607
Shah, V.M., Hardy, B.J., and Stern, S.A., J. Polym. Sci. Phys., 1993, vol. 31, no. 3, pp. 313–317. https://doi.org/10.1002/polb.1993.090310309
Interrante, L.V., Shen, Q., and Li, J., Macromolecules, 2001, vol. 34, pp. 1545–1547. https://doi.org/10.1021/ma001785w
Fritz, G. and Grunert, B., Z. Anorg. Allg. Chem., 1976, vol. 419, pp. 249–252. https://doi.org/10.1002/zaac.19764190305
Borisov, I. L., Ushakov, N. V., Volkov, V. V., and Finkelshtein, E. S., Russ. Chem. Bull., 2016, vol. 65, no. 4, pp. 1020–1022. https://doi.org/10.1007/s11172-016-1406-z
Finkelshtein, E.S., Ushakov, N.V., Krasheninnikov, E.G., and Yampolskii, Y.P., Russ. Chem. Bull., 2004, vol. 53, no. 11, pp. 2604–2610. https://doi.org/10.1007/s11172-005-0161-3.
Eisch, J.J. and Hask, G.R., J. Org. Chem., 1964, vol. 29, pp. 254–256. https://doi.org/10.1021/jo01024a525
Wittenberg, D. and Gilman, H., J. Am. Chem. Soc., 1958, vol. 80, pp. 2677–2680. https://doi.org/10.1021/ja01544a022
Hauser, C.R. and Hance, C.R., J. Am. Chem. Soc., 1952, vol. 74, pp. 5091–5096. https://doi.org/10.1021/ja01140a029
Greber, G. and Metzinger, L., Makromol. Chem., 1960, vol. 39, pp. 226–233. https://doi.org/10.1002/macp.1960.020390116
Fritz, G. and Burdt, H., Z. Anorg. Allg. Chem., 1962, vol. 314, pp. 35–52. https://doi.org/10.1002/zaac.19764190305
Fritz, G. and Burdt, H., Z. Anorg. Allg. Chem., 1962, vol. 317, pp. 35–40. https://doi.org/10.1002/zaac.19623170107
Laskowski, N., Reis, E.-M., Kӧtzner, L., Baus, J. A., Burschka, C., and Tacke, R., Organometallics, 2013, vol. 32, pp. 3269–3278. https://doi.org/10.1021/om400190q
Fisher, M., Burschka, C., and Tacke, R., Organometallics, 2014, vol. 33, pp. 1020–1029. https://doi.org/10.1021/om401208y
Popp, F., Nätscher, J.B., Daiss, J.O., Burschka, C., and Tacke, R., Organometallics, 2007, vol. 26, no. 24, pp. 6014–6028. https://doi.org/10.1021/om700805p
Cho, Y.S., Yoo, B.R., Ahn, S., and Jung, I.N., Bull. Korean Chem. Soc., 1999, vol. 20, no. 4, pp. 427–430.
Kopylov, V.M., Fedotov, A.F., Shkol’nik, M.I., and Raigorodskii, I.M., Zh. Obshch. Khim., 1989, vol. 59, no. 11, pp. 2515–2520.
Patent US 2452895, Publ. 1948.
Bluestein, B.A., J. Am. Chem. Soc., 1948, vol. 70, pp. 3068–3071. https://doi.org/10.1021/ja01189a067
Patent US 2510148, Publ. 1950.
Knoth, W.H., Jr. and Lindsey, R.V., Jr., J. Org. Chem., 1958, vol. 23, no. 9, pp. 1392–1393. https://doi.org/10.1021/jo01103a619
Patent US 2850514, Publ. 1958.
Patent US 2500761, Publ. 1958.
Greber, G. and Degler, G., Makromol. Chem., 1962, vol. 52, pp. 174–183. https://doi.org/10.1002/macp.1962.020520115
Goodwin, J.T.Jr., Boldwin, W.E., and McGregor, R.R., J. Am. Chem. Soc., 1947, vol. 69, p. 2247. https://doi.org/10.1021/ja01201a521
Barrau, J., Hamida, B., and Satge, J., Synth. React. Inorg. Met.-Org. Chem., 1990, vol. 20, no. 10, pp. 1373–1385. https://doi.org/10.1080/00945719008048640
Nametkin, N.S., Islamov, T.H., Gusel’nikov, L.E., and Vdovin, V.M., Russ. Chem. Rev., 1972, vol. 41, pp. 111–130. https://doi.org/10.1070/RC1972v041n02ABEH002056
Andrianov, K.A. and Yakushkina, S.E., Russ. Chem. Bull., 1962, vol. 11, no. 8, pp. 1311–1314. https://doi.org/10.1007/BF00907976
Nametkin, N.S., Gusel’nikov, L.E., Islamov, T.Kh., Shishkina, M.V., and Vdovin, V.M., Dokl. Akad. Nauk SSSR, 1967, vol. 175, no. 1, pp. 136–139.
Patent US 5358670, Publ. 1994.
Patent EP 2086986, Publ. 2009.
Tang, S. and Zhao, H., RSC Adv., 2014, vol. 4, no. 22, pp. 11251–11287. https://doi.org/10.1039/C3RA47191H
ACKNOWLEDGMENTS
The study was performed using the equipment of the Center for Shared Use “Analytical Center for Problems of Deep Oil Refining and Petroleum Chemistry,” Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences.
Funding
The study was financially supported by the Russian Foundation for Basic Research, project no. 18-08-01099.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ushakov, N.V., Finkel’shtein, E.S., Grushevenko, E.A. et al. Synthesis of Monomers for Promising Membrane Materials, Polyalkylenesiloxanes. Russ J Appl Chem 93, 1646–1654 (2020). https://doi.org/10.1134/S1070427220110038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220110038