Abstract
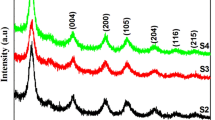
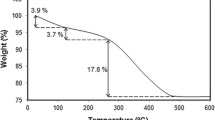
Mesoporous structure of Zn-doped TiO2 as a semiconductor of metal oxide material for high-performance dye-sensitized solar cells (DSSCs) was studied. The Zn-doped TiO2 nanoparticles were synthesized by the colloidal emulsion aphrons (CEAs) method supported by sodium lauryl sulfate (SDS) surfactant with adsorption isotherm type IV and H3-type hysteresis loops. The excellent photoconversion efficiency of 10% Zn-doped TiO2 exhibited the overall improvement of solar cell performance. The 10% Zn-doped TiO2 had a high specific surface area (155 cm3/g). It showed a high photoconversion efficiency of about 6.590% with 28% improvement in the photocurrent density (JSC) compared to undoped TiO2 nanoparticles. There was a reduction of the electron recombination and this synergistically improved the electron mobility and charge collection capability through electrodes in the solar cell.






Similar content being viewed by others
REFERENCES
Grätzel, M., J. Photochem. Photobiol. C Photochem. Rev., 2003, vol. 4, pp. 145–153. https://doi.org/10.1016/S1389-5567(03)00026-1
CHEN, X., Chin. J. Catal., 2009, vol. 30, pp. 839–851. https://doi.org/10.1016/S1872-2067(08)60126-6
Hagfeldt, A., Boschloo, G., Sun, L., Kloo, L., and Pettersson, H., Chem. Rev., 2010, vol. 110, pp. 6595–6663. https://doi.org/10.1021/cr900356p
Xu, H., Tao, X., Wang, D.-T., Zheng, Y.-Z., and Chen, J.-F., Electrochimica Acta, 2010, vol. 55, pp. 2280–2285. https://doi.org/10.1016/j.electacta.2009.11.067
Gao, Z., Wu, Z., Li, X., Chang, J., Wu, D., Ma, P., Xu, F., Gao, S., and Jiang, K., Cryst. Eng. Comm., 2013, vol. 15, pp. 3351–3358. https://doi.org/10.1039/C3CE27098J
An’amt, M.N., Radiman, S., Huang, N.M., Yarmo, M.A., Ariyanto, N.P., Lim, H.N., and Muhamad, M.R., Ceram. Int., 2010, vol. 36, pp. 2215–2220. https://doi.org/10.1016/j.ceramint.2010.05.027
Khan, M.A., Shaheer Akhtar, M., and Yang, O.-B., Sol. Energy., 2010, vol. 84, pp. 2195–2201. https://doi.org/10.1016/j.solener.2010.08.008
Muniz, E.C., Góes, M.S., Silva, J.J., Varela, J.A., Joanni, E., Parra, R., and Bueno, P.R., Ceram. Int., 2011, vol. 37, pp. 1017–1024. https://doi.org/10.1016/j.ceramint.2010.11.014
Henrist, C., Dewalque, J., Mathis, F., and Cloots, R., Microporous Mesoporous Mater., 2009, vol. 117, pp. 292–296. https://doi.org/10.1016/j.micromeso.2008.07.001
Crossland, E.J.W., Nedelcu, M., Ducati, C., Ludwigs, S., Hillmyer, M.A., Steiner, U., and Snaith, H.J., Nano Lett., 2009, vol. 9, pp. 2813–2819. https://doi.org/10.1021/nl800942c
Hung, I.-M., Wang, Y., Huang, C.-F., Fan, Y.-S., Han, Y.-J., and Peng, H.-W., J. Eur. Ceram. Soc., 2010, vol. 30, pp. 2065–2072. https://doi.org/10.1016/j.jeurceramsoc.2010.04.015
Pan, J.H., Zhao, X.S., and Lee, W.I., Environ. Nanotechnol., 2011, vol. 170, pp. 363–380. https://doi.org/10.1016/j.cej.2010.11.040
Jamwal, D., Kaur, G., Raizada, P., Singh, P., Pathak, D., and Thakur, P., J. Phys. Chem. C., 2015, vol. 119, pp. 5062–5073. https://doi.org/10.1021/jp510428z
Platz, G., Adv. Mater., 1989, vol. 1, pp. 94–95. https://doi.org/10.1002/adma.19890010312
Dai, Y. and Deng, T., J. Colloid Interface Sci., 2003, vol. 261, pp. 360–365. https://doi.org/10.1016/S0021-9797(03)00056-0
Jauregi, P., Gilmour, S., Varley, J., Chem. Eng. J., 1997, vol. 65, pp. 1–11. https://doi.org/10.1016/S1385-8947(96)03154-3
Roy, D., Valsaraj, K.T., and Kottai, S.A., Sep. Sci. Technol., 1992, vol. 27, pp. 573–588. https://doi.org/10.1080/01496399208018903
Deng, T., Dai, Y., and Wang, J., Colloids Surf. Physicochem. Eng. Asp., 2005, vol. 266, pp. 97–105. https://doi.org/10.1016/j.colsurfa.2005.05.067
Yan, Y., Zhang, N., and Qu, C., Colloids Surf. Physicochem. Eng. Asp., 2005, vol. 264, pp. 139–146. https://doi.org/10.1016/j.colsurfa.2005.04.025
Supakanapitak, S., Boonamnuayvitaya, V., and Jarudilokkul, S., Mater. Charact., 2012, vol. 67, pp. 83–92. https://doi.org/10.1016/j.matchar.2012.02.018
Dai, Y., Deng, T., Jia, S., Jin, L., and Lu, F., J. Membr. Sci., 2006, vol. 281, pp. 685–691. https://doi.org/10.1016/j.memsci.2006.04.039
Chen, C., Li, X., Ma, W., Zhao, J., Hidaka, H., and Serpone, N., J. Phys. Chem. B., 2002, vol. 106, pp. 318–324. https://doi.org/10.1021/jp0119025
Li, G., Zhang, D., and Yu, J.C., Phys. Chem. Chem. Phys., 2009, vol. 11, pp. 3775–3782. https://doi.org/10.1039/B819167K
Li, M., Zhang, S., Lv, L., Wang, M., Zhang, W., and Pan, B., Chem. Eng. J., 2013, vol. 229, pp. 118–125. https://doi.org/10.1016/j.cej.2013.05.106
Liu, Y., Sun, X., Tai, Q., Hu, H., Chen, B., Huang, N., Sebo, B., and Zhao, X., J. Power Sources., 2011, vol. 196, pp. 475–481. https://doi.org/10.1016/j.jpowsour.2010.07.031
Al-juaid, F., Merazga, A., AL-Baradi, A., and Abdel-Wahab, F., Solid State Electron., 2013, vol. 87, pp. 98–103. https://doi.org/10.1016/j.sse.2013.06.007
Hossein Habibi, M., Askari, E., Habibi, M., and Zendehdel, M., Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 2013, vol. 104, pp. 197–202. https://doi.org/10.1016/j.saa.2012.11.055
Zywitzki, D., Jing, H., Tüysüz, H., and Chan, C.K., J. Mater. Chem. A., 2017, vol. 5, pp. 10957–10967. https://doi.org/10.1039/C7TA01614
ACKNOWLEDGMENTS
Financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (grant no. PHD/0110/2553) to Thanawat Buapuean and Somnuk Jarudilokkul is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Buapuean, T., Jarudilokkul, S. Synthesis of Mesoporous Zn-doped TiO2 Nanoparticles by Colloidal Emulsion Aphrons and Their Use for Dye-sensitized Solar Cells. Russ J Appl Chem 93, 1229–1236 (2020). https://doi.org/10.1134/S1070427220080169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220080169