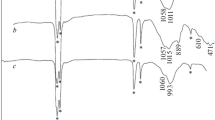
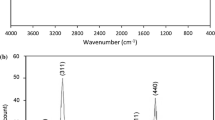
Abstract
Two approaches were considered in searching for an efficient procedure for preparing magnetic nanocomposites based on activated carbon and magnetite nanoparticles by chemical precipitation: preliminary synthesis of magnetite nanoparticles by chemical precipitation from solutions of bi- and trivalent iron salts, followed by introduction of the nanoparticles into the activated carbon matrix, and synthesis of magnetite nanoparticles in the activated carbon matrix. A comparative analysis of the content of magnetite nanoparticles and functional characteristics (textural parameters, sorption capacity, saturation magnetization, and coercive force of the nanocomposites) was made. The ex situ synthesis proved to be the best procedure for preparing nanocomposite sorbents based on activated carbon and magnetite nanoparticles by chemical precipitation, as judged from the yield and functional characteristics of the target product. In biological testing, the maximal harmless (inactive) concentration of the magnetic nanocomposite in the test system with microalgae appeared to be higher by an order of magnitude than that in the test system with ciliates.






Similar content being viewed by others
REFERENCES
Li, X., Xu, J., Jiang, G., and Xu, X., Chemosphere, 2011, vol. 85, no. 7, pp. 1204–1209. https://doi.org/10.1016/j.chemosphere.2011.09.005
Li, X., Xu, J., Jiang, G., Tang, J., and Xu, X., J. Colloid Interface Sci., 2012, vol. 369, pp. 460–469. https://doi.org/10.1016/j.jcis.2011.11.049
Kalantry, R.R., Jafari, A.J., Esrafili, A., Kakavandi, B., Gholizadeh, A., and Azari, A., Desalinat. Water Treat., 2016, vol. 57, no. 14, pp. 6411–6422. https://doi.org/10.1080/19443994.2015.1011705
Teresa, J.B., Activated Carbon Surfaces in Environmental Remediation, New York: Elsevier, 2006, p. 572
Wong, K.T., Nguk, C.E., Shaliza, I., Hyunook, K., Yeomin, Y., and Min, J., J. Cleaner Prod., 2015, vol. 115, pp. 337–342. https://doi.org/10.1016/j.jclepro.2015.12.063
Altintig, E., Altundag, H., Tuzen, M., and Sari, A., Chem. Eng. Res. Des., 2017, vol. 122, pp. 151–163. https://doi.org/10.1016/j.cherd.2017.03.035
Altintig, E., Onaran, M., Sari, A., Altundag, H., and Tuzen, M., Mater. Chem. Phys., 2018, vol. 220, pp. 313–321. https://doi.org/10.1016/j.matchemphys.2018.05.077
Gautam, R.K., Banerjee, V.R.S., Sanroman, M.A., and Singh, S.S.K., J. Mol. Liq., 2015, vol. 232, pp. 227–236. https://doi.org/10.1016/j.molliq.2015.09.006
Kakavandi, B., Kalantary, R.R., Ahmadi, E., Gholami, Torkshavand, Z., and Azizi, M., J. Porous Mater., 2015, vol. 22, no. 4, pp. 1083–1096. https://doi.org/10.1007/s10934-015-9983-z
Gu, S., Hsieh, C., Gandom, Y.A., Yang, Z., Li, L., Fu, C., and Juang, R., J. Mol. Liq., 2018, vol. 277, pp. 499–505. https://doi.org/10.1016/j.molliq.2018.12.018
Baghdadi, M., Ghaffari, E., and Aminzadeh, B., J. Environ. Chem. Eng., 2016, vol. 4, no. 3, pp. 3309–3321. https://doi.org/10.1016/j.jece.2016.06.034
Badi, M.Y., Azari, A., Pasalari, H., Esrafili, A., and Farzadkia, M., J. Mol. Liq., 2018, vol. 261, pp. 146–154. https://doi.org/10.1016/j.molliq.2018.04.019
Pomogailo, A.D. and Dzhardimalieva, G.I., Metallo-polimernye gibridnye nanokompozity (Metal–Polymer Hybrid Nanocomposites), Moscow: Nauka, 2015.
Karnaukhov, A.P., Adsorbtsiya. Tekstura dispersnykh i poristykh materialov (Adsorption. Texture of Disperse and Porous Materials), Novosibirsk: Nauka, 1993.
Funding
The study was supported by the Russian Foundation for Basic Research (project no. 19-33-90149). The magnetic properties of the samples were studied by G.I. Dzhardimalieva within the theme of the government assignment (state registry no. 0089-2029-0012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bondarenko, L.S., Magomedov, I.S., Terekhova, V.A. et al. Magnetite–Activated Carbon Nanocomposites: Synthesis, Sorption Properties, and Bioavailability. Russ J Appl Chem 93, 1202–1210 (2020). https://doi.org/10.1134/S1070427220080133
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220080133