Abstract
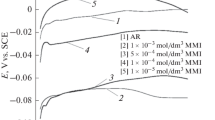
A series of 3-(N-arylamino)-5-amino-1Н-1,2,4-triazoles were prepared by the reaction of S,S-dimethyl cyanodithioimidocarbamate with appropriate anilines. The inhibiting effect of the products on the corrosion of copper of M1 grade in acidic and neutral chloride-containing media was studied using electrochemical and accelerated electroless methods. The maximal degree of protection ensured by commercially available 3,5-diamino-1H-1,2,4-triazole and the synthesized 3-phenylamino, 3-(4-methylphenylamino), 3-(4-chlorophenylamino), and 3-(3-chlorophenylamino) derivatives of 5-amino-1Н-1,2,4-triazole reaches 70–87%. In acid solutions, the synthesized compounds show higher performance than the 3,5-diamino derivative, ensuring up to 92–96% protection. Similar results were obtained in experiments in a salt fog chamber.





Similar content being viewed by others
REFERENCES
Kear, G., Barker, B.D., and Walsh, F.C., Corros. Sci., 2004, vol. 46, pp. 109–135. https://doi.org/10.1016/s0010-938x(02)00257-3
Finsgar, M. and Milosev, I., Corros. Sci., 2010, vol. 52, pp. 2737–2749. https://doi.org/10.1016/j.corsci.2010.05.002
Khiati, Z., Othman, A.A., Sanchez-Moreno, M., Bernard, M.-C., Joiret, S., Sutter, E.M.M., and Vivier, V., Corros. Sci., 2011, vol. 53, pp. 3092–3099. https://doi.org/10.1016/j.corsci.2011.05.042
Finšgar, M., Corros. Sci., 2013, vol. 77, pp. 350–359. https://doi.org/10.1016/j.corsci.2013.08.026
Skrypnikova, E.A., Kaluzhina, S.A., and Agafonova, L.E., Int. J. Corros. Scale Inhib., 2014, vol. 3, no.1, pp. 59–65. https://doi.org/10.17675/2305-6894-2014-3-1-059-065
Dugdale, I. and Cotton, J.B., Corros. Sci., 1963, vol. 2, no. 1, pp. 69–75. https://doi.org/10.1016/s0010-938x(63)80001-3
Poling, G.W., Corros. Sci., 1970, vol. 10, pp. 359–370. https://doi.org/10.1016/s0010-938x(70)80026-9
Es-Salah, K., Keddam, M., Rahmouni, K., Srhiri, A., and Takenouti, H., Electrochim. Acta, 2004, vol. 49, nos. 17–18, pp. 2771–2778. https://doi.org/10.1016/j.electacta.2004.01.038
Moussa, M.N.H., El-Far, A.A., and El-Shafei, A.A., Mater. Chem. Phys., 2007, vol. 105, pp. 105–113. https://doi.org/10.1016/j.matchemphys.2007.04.007
Rivera-Grau, L.M., Casales, M., Regla, I., Ortega-Toledo, D.M., Ascencio-Gutierrez, J.A., Porcayo-Calderon, J., and Martinez-Gomez, L., Int. J. Electrochem. Sci., 2013, vol. 8, pp. 2491–2503.
Wittenbrook, L.S., Smith, G.L., and Timmons, R.J., J. Org. Chem., 1973, vol. 38, no. 3, pp. 465–471. https://doi.org/10.1021/jo00943a011
Mansfeld, F., in Advances in Corrosion Science and Technology, Boston, MA: Springer, 1976. https://doi.org/10.1007/978-1-4684-8986-6_3
Mansfeld, F., Corros. Sci., 2005, vol. 47, no. 12, pp. 3178–3186. https://doi.org/10.1016/j.corsci.2005.04.012
Shih, H. and Mansfeld, F., in Computer Modeling in Corrosion, ASTM International, 1992, pp. 174–185. https://doi.org/10.1520/STP24695S
Funding
Studies on the synthesis of 3-(N-arylamino)-5-amino-1Н-1,2,4-triazoles and on optimization of the process conditions were supported by the Ministry of Education and Science of the Russian Federation within the framework of the government assignment for higher schools in the sphere of scientific activity for the years 2017–2019, project no. 4.3633.2017/4.6.
Electrochemical and electroless corrosion tests were financially supported by the Science and Engineering Research Board (SERB, New Delhi, India, grant SB/FT/CS-101/2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shevtsov, D.S., Shikhaliev, K.S., Komarova, E.S. et al. Inhibition of Copper Corrosion with N-Arylaminotriazoles in Aqueous Chloride Solutions and in Air. Russ J Appl Chem 93, 1152–1159 (2020). https://doi.org/10.1134/S1070427220080078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220080078