Abstract
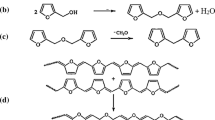
Properties of pyrolyzates with the developed porous structure, prepared in the system consisting of phenol–formaldehyde resol resin and ethylene glycol after polymerization induced phase separation (PIPS), post-curing, and pyrolysis, were studied. The structure formed in the PIPS step is determined by the reactivity of the thermosetting component. With an increase in the rate of curing of the resol component, at equal PIPS temperature, the macropore size decreases and the cumulative volume of mesopores increases. An increase in the curing rate leads to an increase in the separation intensity and to a decrease in the degree of microphase separation. The pyrolysis yields an X-ray amorphous material with small inclusions of the crystalline graphite phase.




Similar content being viewed by others
REFERENCES
Dospekhi dlya “BURANA.” Materialy i tekhnologii VIAM dlya MKS “ENERGIYa-BURAN” (Armor for BURAN. Materials and Technologies of the All-Russia Research Institute of Aviation Materials for ENERGIYa-BURAN International Space Station), Kablov, E.N., Ed., Moscow: Fond Nauka i Zhizn, 2013.
Kablov, E.N., Grashchenkov, D.V., Isaeva, N.V., Solntsev, S.S., and Sevast’yanov, V.G., Glass Ceram., 2012, vol. 69, nos. 3–4, pp. 109–112. https://doi.org/10.1007/s10717-012-9425-1
Kablov, E.N., Grashchenkov, D.V., Isaeva, N.V., and Solntsev, S.S., Ross. Khim. Zh., 2010, vol. 54, no. 1, pp. 20–24.
Prokof’ev, V.A., Sorokin, O.Yu., Vaganova, M.L., and Lebedeva, Yu.E., Tr. Vseross. Nauch.-Issled. Inst. Aviats. Mater.: Elektron. Nauch.-Tekh. Zh., 2018, no. 11, article 06. https://doi.org/10.18577/2307-6046-2018-0-11-45-53
Nam, G., Choi, S., Byun, H., Rhym, Y.-M., and Shim, S.E., Macromol. Res., 2013, vol. 21, no. 9, pp. 958–964. https://doi.org/10.1007/s13233-013-1114-6
Nishihara. H., Mukai. S.R., and Tamon, H., Carbon, 2004, vol. 42, pp. 885–901. https://doi.org/10.1016/j.carbon.2004.01.075
Horikawa, T., Ogawa, K., Mizuno, K., Hayashi, J., and Muroyama, K., Carbon, 2003, vol. 41, pp. 465–472. https://doi.org/10.1016/S0008-6223(02)00352-4
Khaskov, M.A., Maklakov, S.S., Filenko, D.G., Stupnikova, T.V., and Avdeev, V.V., Vestn. Mosk. Univ., Ser. 2: Khimiya, 2006, vol. 47, no. 5, pp. 323–326.
Khaskov, M.A., Shestakov, A.M., Sinyakov, S.D., Sorokin, O.Yu., Gulyaev, A.I., and Zelenina, I.V., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2018, vol. 61, no. 11, pp. 31–37. https://doi.org/10.6060/ivkkt.20186111.3y
Zhang, G., Liu, G., Shi, Z., and Qiao, G., RSC Adv., 2014, vol. 4, pp. 7068–7078. https://doi.org/10.1039/c3ra46490c
Zhang, G. and Qiao, G., J. Chem. Phys., 2013, vol. 139, no. 13, p. 134903. https://doi.org/10.1063/1.4822295
Zhang, G., Xiao, Z., and Qiao, G., Key Eng. Mater., 2012, vol. 512–515, pp. 403–406. https://doi.org/10.4028/www.scientific.net/KEM.512-515.403
Wu, X., Zhu, Y., Pei, B., Cai, P., and Huang, Z., Mater. Lett., 2018, vol. 215, pp. 50–52. https://doi.org/10.1016/j.matlet.2017.12.049
Xu, S., Qiao, G., Wang, H., Li, D., and Lu, T., Mater. Lett., 2008, vol. 62, nos. 21–22, pp. 3716–3718.. https://doi.org/10.1016/j.matlet.2008.04.
Phenolic Resins. Chemistry, Applications, Standardization, Safety and Ecology, Gardziella, A., Pilato, L.A., and Knop,A., Eds., Berlin: Springer
Monni, J., Alvila, L., and Pakkanen, T.T., Ind. Eng. Chem., 2007, vol. 46, pp. 6916–6924. https://doi.org/10.1021/ie070297a
Patent US Publ. 2000
Riikonen, J., Salonen, J., and Lehto, V.-P., J. Therm. Anal. Calorim., 2011, vol. 105, pp. 823–830. https://doi.org/10.1007/s10973-011-1337-8
Satdinov, R.A., Istyagin, S.E., and Veshkin, E.A., Tr. Vseross. Nauch.-Issled. Inst. Aviats. Mater.: Elektron. Nauch.-Tekh. Zh., 2017, no. 3, article 09. https://doi.org/10.18577/2307-6046-2017-0-3-9-9
Rozenberg, B.A., Ross. Khim. Zh., 2001, vol. XLV, nos. 5–6, pp. 23–31.
Martinez, I., Martin, M.D., Eceiza, A., Oyanguren, P., and Mondragona, I., Polymer, 2000, vol. 41, pp. 1027–1035. https://doi.org/10.1016/S0032-3861(99)00238-4
Peng, L., Cui, J., and Li, S., Macromol. Chem. Phys., 2000, vol. 201, pp. 699–704.
Wang, M., Yu, Y., Wu, X., and Li, S., Polymer, 2004, vol. 45, pp. 1253–1259. https://doi.org/10.1016/j.polymer.2003.12.037
Swier, S. and Van Mele, B., Polymer, 2003, vol. 44, pp. 2689–2699. https://doi.org/10.1016/S0032-3861(03)00138-1
Khaskov, M.A., Polym. Sci., Ser. B, 2017, vol. 59, no. 1, pp. 51–61. https://doi.org/10.1134/S1560090417010080
Khaskov, M.A., Gulyaev, A.I., Sinyakov, S.D., and Ponomarenko, S.A., Mater. Chem. Phys., 2019, vol. 233, pp. 236–241. https://doi.org/10.1016/j.matchemphys.2019.05.060
Oh, J. and Rey, A.D., Comput. Theor. Polym. Sci., 2001, vol. 11, pp. 205–217. https://doi.org/10.1016/S1089-3156(00)00013-1
Valueva, M.I., Zelenina, I.V., Khaskov, M.A., and Gulyaev, A.I., Tr. Vseross. Nauch.-Issled. Inst. Aviats. Mater.: Elektron. Nauch.-Tekh. Zh., 2017, no. 10, article 09. https://doi.org/10.18577/2307-6046-2017-0-10-9-9
Khaskov, M.A., Shestakov, A.M., Sorokin, O.Yu., Gulyaev, A.I., Davydova, E.A., Sulyanova, E.A., Sinyakov, S.D., Valueva, M.I., and Zelenina, I.V., Mater. Today: Proc., 2018, vol. 5, no. 12(3), pp. 26046–26051. https://doi.org/10.1016/j.matpr.2018.08.027
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
FUNDING
The study was financially supported by the Russian Foundation for Basic Research (project no. 17-03-01163).
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khaskov, M.A., Davydova, E.A., Valueva, M.I. et al. Influence of the Reactivity of the Thermosetting Component in the Resol Resin/Ethylene Glycol System on the Properties of Pyrolyzates. Russ J Appl Chem 93, 204–211 (2020). https://doi.org/10.1134/S107042722002007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042722002007X