Abstract
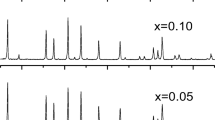
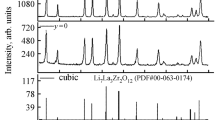
Solid electrolytes Li7−xLa3Zr2−xNbxO12 (x = 0.0–2.0) were synthesized by the sol-gel method, with Nb2O5 used as one of the starting compounds. The suggested synthesis procedure made it possible to lower the annealing temperature and shorten the annealing duration as compared with the solid-phase synthesis method, from 1200°C during 36 h to 1150°C during 1 h. The influence exerted by the doping of the Li7La3Zr2O12 compound in the Zr sublattice with niobium (Nb5+) on its crystal structure, morphology, and electrical conductivity. It was found that the compounds obtained with x > 0.1 are single-phase and have the Ia−3d cubic structure. The scanning electron microscopy was used to examine the morphology and the grain size of the resulting solid electrolytes. The average grain size of the ceramic samples was found to be 1–4 µm. The resistivity of the solid electrolytes Li7−xLa3Zr2−xNbxO12 (x = 0.0–2.0) was measured by the method of electrochemical impedance. It was found that Li6.75La3Zr1.75Nb0.25O12 has the highest total lithium-ion conductivity at 25°C: 4.0 × 10−5 S cm−1. It was shown that the sol-gel method is promising and can be used to obtain solid electrolytes based on Li7La3Zr2O12 doped with Nb5+ ions.
Similar content being viewed by others
References
Ramakumar, S., Deviannapoorani, C., Dhivya, L., Shankar, L.S., and Murugan, R., Prog. Mater. Sci., 2017, vol. 88, pp. 325–411. https://doi.org/10.1016/j.pmatsci.2017.04.007
Yaroslavtsev, A.B., Russ. Chem. Rev., 2016, vol. 85, pp. 1255–1276. https://doi.org/10.1070/RCR4634
Kammampata, S.P. and Thangadurai, V., Ionics, 2018, vol. 24, pp. 639–660. https://doi.org/10.1007/s11581-017-2372-7
Liu, Q., Geng, Z., Han, C., Fu, Y., Li, S., He, Y., and Li, B., J. Power Sources, 2018, vol. 389, pp. 120–134. https://doi.org/10.1016/j.jpowsour.2018.04.019
Zheng, F., Kotobuki, M., Song, S., Lai, M.O., and Lu, L., J. Power Sources, 2018, vol. 389, pp. 198–213. https://doi.org/10.1016/j.jpowsour.2018.04.022
Hyooma, H. and Hayashi, K., Mater. Res. Bull., 1988, vol. 23, pp. 1399–1407. https://doi.org/10.1016/0025-5408(88)90264-4
Mazza, D., Mater. Lett., 1988, vol. 7, pp. 205–207. https://doi.org/10.1016/0167-577X(88)90011-0
Thangadurai, V., Kaack, H., and Weppner, W., J. Am. Ceram. Soc., 2003, vol. 86, pp. 437–440. https://doi.org/10.1111/j.1151-2916.2003.tb03318.x
Murugan, R., Thangadurai, V., and Weppner, W., Angew. Chem., Int. Ed., 2007, vol. 46, pp. 7778–7781. https://doi.org/10.1002/anie.200701144
Raskovalov, A.A., Il’ina, E.A., and Antonov, B.D., J. Power Sources, 2013, vol. 238, pp. 48–52. https://doi.org/10.1016/j.jpowsour.2013.03.049
Li, Y., Han, J., Wang, C.-A., Vogel, S.C., Xie, H., Xu, M., and Goodenough, J.B., J. Power Sources, 2012, vol. 209, pp. 278–281. https://doi.org/10.1016/j.jpowsour.2012.02.100
Bernuy-Lopez, C., Manalastas, W., Lopez del Amo, J.M., Aguadero, A., Aguesse, F., and Kilner, J.A., Chem. Mater., 2014, vol. 26, pp. 3610–3617. https://doi.org/10.1021/cm5008069
Li, Y., Han, J.-T., Wang, C.-A., Xie, H., and Goodenough, J.B., J. Mater. Chem., 2012, vol. 22, pp. 15357–15361. https://doi.org/10.1039/c2jm31413d
Ohta, S., Kobayashi, T., and Asaoka, T., J. Power Sources, 2011, vol. 196, pp. 3342–3345. https://doi.org/10.1016%2Fj.jpowsour.2010.11.089
Zhao, P., Xiang, Y., Wen, Y., Li, M., Zhu, X., Zhao, S., Jin, Z., Ming, H., and Cao, G., J. Eur. Ceram. Soc., 2018, vol. 38, pp. 5454–5462. https://doi.org/10.1016/jjeurceramsoc.2018.08.037
Il’ina, E.A., Andreev, O.L., Antonov, B.D., and Batalov, N.N., J. Power Sources, 2012, vol. 201, pp. 169–173. https://doi.org/10.1016/jjpowsour.2011.10.108
Li, Y., Yang, T., Wu, W., Cao, Z., He, W., Gao, Y., Liu, J., and Li, G., Ionics, 2018, vol. 24, pp. 3305–3315. https://doi.org/10.1007/s11581-018-2497-3
Rosero-Navarro, N.C., Yamashita, T., Miura, A., Higuchi, M., and Tadanaga, K., Solid State Ionics, 2016, vol. 285, pp. 6–12. https://doi.org/10.1016/j.ssi.2015.06.015
Sherstobitova, E.A., Gubkin, A.F., Bobrikov, I.A., Kalashnova, A.V., and Pantyukhina, M.I., Electrochim. Acta, 2016, vol. 209, pp. 574–581. https://doi.org/10.1016/j.electacta.2016.05.113
Rettenwander, D., Redhammer, G., Preishuber-Pflugl, F., Cheng, L., Miara, L., Wagner, R., Welzl, A., Suard, E., Doeff, M.M., Wilkening, M., Fleig, J., and Amthauer, G., Chem. Mater., 2016, vol. 28, no. 7, pp. 2384–2392. https://doi.org/10.1021/acs.chemmater.6b00579
Acknowledgments
The materials obtained in the study were certified on the equipment of the Collective Use Center “Substance composition” at the Institute of High-Temperature Electrochemistry, Ural Branch, Russian Academy of Science.
Funding
The study was financially supported by the RF Presidential grant no. MK-1382.2019.3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that there is no conflict of interest to be disclosed in the present communication.
Additional information
Russian Text © The Author(s), 2019, published in Zhurnal Prikladnoi Khimii, 2019, Vol. 92, No. 12, pp. 1543–1549.
Rights and permissions
About this article
Cite this article
Il’ina, E.A., Lyalin, E.D., Antonov, B.D. et al. Lithium-conducting Solid Electrolytes Synthesized by the Sol-Gel Method in the System Li7La3Zr2O12-Li5La3Nb2O12. Russ J Appl Chem 92, 1657–1663 (2019). https://doi.org/10.1134/S107042721912005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042721912005X