Abstract
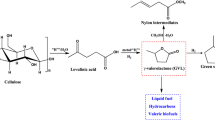
In this report, catalysts of Au nanoparticles on different supports (Au/ZrO2, Au/C, Au/Al2O3, Au/SiO2, Au/TiO2, Au/MgO) were fabricated by co-precipitation and impregnation methods to determine the role of Au over oxides. The crystal structure and phase composition of catalyst samples before and after test reactions were investigated by X-ray diffraction technique, X-ray photoelectron spectroscopy and transmission electron microscopy. The catalytic activity was tested on the hydrogenation reaction of levulinic acid (LA) into γ-valerolactone (GVL) using formic acid (FA) as a hydrogen source. In all tested samples, Au/ZrO2-D (was fabricated by co-precipitation method) gave the best GVL yield of 85.0% with very low amount of catalyst loading (catalyst/reactant 1 wt). The existent of Au3+ in the catalyst system may be the main factor to improve the yield of GVL formation.
Similar content being viewed by others
References
Escobar, J.C., Lora, E.S., Venturini, O.J., et al., Renew. Sustain. Energy Rev., 2009, vol. 13, pp. 1275-1287; Hu, L., Zhao, G., Hao, W., et al., RSC Adv., 2012, vol. 2, pp. 11184-11206; Corma, A., Iborra, S., and Velty, A., Chem. Rev., 2007, vol. 107, pp. 2411–2502.
Alonso, D.M., Bond, J.Q., and Dumesic, J.A., Green Chem., 2010, vol. 12, pp. 1493–1513. Huber, G.W., Iborra, S., and Corma. A., Chem. Rev., 2006, vol. 106, pp. 4044–4098; Takagaki, A., Nishimura, S., and Ebitani, K., Catal. Surv. Asia, 2012, vol. 16, pp. 164–182.
Nigam, P.S. and Singh, A., Combust. Sci., 2011, vol. 37, pp. 52–68; Gallezot, P., Chem. Soc. Rev., 2012, vol. 41, pp. 1538–1558.
Huber, G.W. and Corma, A., Chem. Rev., 2006, vol. 106, pp. 4044–4098.
Kamm, B., Angew. Chem. Int. Ed., 2007, vol. 46, pp. 5056–5058.
Coombs, J. and Hall, K., Renew. Energy, 2007, vol. 15, pp. 54–59.
Horvath, I.T., Mehdi, H., Fabos, V., Boda, L., and Mika, L.T., Green Chem., 2008, vol. 10, pp. 238–242.
Savage, N., Nature, 2011, vol. 474, S9–S11.
Bond, J.Q., Alonso, D.M., Wang, D., West, R.M., and Dumesic, J.A., Science, 2010, vol. 327, pp. 1110–1111; Bond, J.Q., Alonso, D.M., West, R.M., and Dumesic, J.A., Langmuir, 2010, vol. 26, pp. 16291–16298.
Deng, J., Wang, Y., Pan, T., Xu, Q., et al., Chem. Sus. Chem., 2013, vol. 6, pp. 1163–1167.
Dunlop, A.P. and Madden, J.W., US patent 2786852, 1957.
Alonso, D.M., Wettstein, S.G., and Dumesic, J.A., Green Chem., 2013, vol. 15, pp. 584–595.
Wettstein, S.G., Alonso, D. M., Chong, Y., and Dumesic, J.A., Energy Environ. Sci., 2012, vol. 5, pp. 8199–8203.
Yan, K., Liao, J., Wu, X., and Xie, X., RSC Adv., 2013, vol. 3, pp. 3853–3856.
Sen, S.M., Alonso, D.M., Wettstein, S.G., et al., Energy Environ. Sci., 2012, vol. 5, pp. 9690–9697.
Zhang, J., Wu, S.B., Li, B., and Zhang, H.D., Chem. Cat. Chem., 2012, vol. 4, pp. 1230–1237.
Manzer, L.E., Appl. Catal., A, 2004, vol. 272, pp. 249–256; Al-Shaal, M.G., William, W.R.H., and Palkovits, R., Green Chem., 2012, vol. 14, pp. 1260–1263.
Yan, Z., Lin, L., and Liu, S., Energy Fuels, 2009, vol. 23, pp. 3853–3858.
Upare, P.P., Lee, J.M., Hwang, D.W., Halligudi, S.B., et al., J. Ind. Eng. Chem., 2011, vol. 17, pp. 287–292.
Lv, J., Rong, Z., Wang, Y., et al., RSC Adv., 2015, vol. 5, pp. 72037–2045.
Li, W., Xie, J.H., Lin, H., and Zhou, Q.L., Green Chem., 2012, vol. 14, pp. 2388–2390.
Wright, W.R.H. and Palkovits, R., Chem. Sus. Chem., 2012, vol. 5, pp. 1657–1667.
Wang, J., Jaenicke, S., and Chuah, G.-K., RSC Adv., 2014, vol. 4, pp. 13481–13489; Kuwahara, Y., Kaburagi, W., Osada, Y., Fujitani, T., and Yamashita, H., Catal. Today, 2017, vol. 281, pp. 418–428.
He, J., Li, H., Lu, Y.-M., et al., Appl. Catal. A: Gen., 2016, vol. 510, pp. 11-19; Li, H., Fang, Z., and Yang, S., ACS Sustain. Chem. Eng., 2016, vol. 4, pp. 236–246.
Li, H., Fang, Z., and Yang, S., Chem. Plus. Chem., 2016, vol. 81, pp. 135–142.
Du, X.-L., He, L., Liu, Y.-M., et al., Angew. Chem. Int. Ed., 2011, vol. 50, pp. 7815–7819.
Pham, S.A., Shun, N., and Kohki, E., RSC Adv., 2014, vol. 4, pp. 10525–10530.
Acknowledgments
This research is funded by the Vietnam National University, Hanoi (VNU) under project number QG.15.16.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors state that they have no conflict of interest to be disclosed in the present communication.
Rights and permissions
About this article
Cite this article
Son, P.A., Hoang, D.H. & Canh, K.T. The Role of Gold Nanoparticles on Different Supports for the In-Air Conversion of Levulinic Acid into γ-Valerolactone with Formic Acid as an Alternative Hydrogen Source. Russ J Appl Chem 92, 1316–1323 (2019). https://doi.org/10.1134/S1070427219090179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427219090179