Abstract
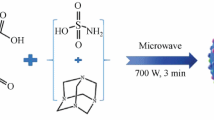
In this paper, citric acid was used as carbon source, diethylenetriamine and urotropine were used as precursors, and N-doped carbon dots (cdh-CDs) with luminescent properties were synthesized by microwave method. The structure of the sample was analyzed by Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), X-ray diffractometry (XRD), ultraviolet spectrophotometer (UV), fluorescence spectroscopy, etc., and it was proved that cdh-CDs with an amorphous structure about 4–10 nm in diameter was synthesized. The corrosion inhibition performance of cdh-CDs in 1 M hydrochloric acid was evaluated by static weight loss and electrochemical method. The results showed that cdh-CDs have a good corrosion inhibition performance at 60°C, the inhibition efficiency can reach 81.2% when the dosage is 600 ppm. Electrochemical results showed that cdh-CDs are mixed inhibitors which mainly inhibit cathodes.
Similar content being viewed by others
References
Ortega, D.M., Materials Chemistry & Physics, 2010, 122, vol. 2, p. 485–490.
Stalheim, D.G. and Douglas, G., Metallurgical Optimization of Microalloyed Steels for Oil and Gas Transmission Pipelines, Int. Conf. on High Strength Low Alloy Steels, Beijing, 2011.
Stalheim, D.G., The Use of High Temperature Processing (HTP) Steel for High Strength Oil and Gas Transmission Pipeline Applications, Int. Conf. on High Strength Low Alloy Steels, Beijing, 2005.
Vengosh, A., Jackson, R.B., Warner, N., et al., Env. Sci. & Techn., 2014, vol. 48, no. 15, pp. 8334–8348.
Vidic, R.D., Brantley, S.L., Vandenbossche, J.M., et al., Science, 2013, vol. 340, no. 6134, pp. 1235009–1235009.
Murphy, T., Kelly, S., Ahmad, K., SSRN Electronic Journal, 2015, vol. 49, no. 7, pp. 553–557.
House, K.Z., Harvey, C.F., Aziz, M.J., et al. Env. Sci. & Techn., vol. 2, no. 2, pp. 193–205.
Wahbi, Z., Guenbour, A., Makarim, H.A.E., et al., Progress in Organic Coatings, 2007, vol. 60, no. 3, pp. 224–227.
Kassim, J. and Rahim, A.A., Recent Patents on Materials Science, 2008, vol. 1, no. 3, pp. 18–56.
Glossman-Mitnik, D., J. Molecular Structure THEOCHEM., 2004, vol. 681, nos. 1–3, pp. 83–88.
Das, R., Bandyopadhyay, R., and Pramanik, P., Carbon Quantum Dots from Natural Resource: A Review, 2018, 8, pp. 96–109.
Ma, B., Zhang, S., Liu, R., et al., Nanoscale, 2017, vol. 9, no. 6, pp. 2162–2171.
Wang, W., Lu, Y.C., Huang, H., et al., The Analyst, 2014, vol. 139, no. 7, pp. 1692–1696.
Huang, P, Lin, J., Wang, X., et al., Advanced Materials, 2012, vol. 24, no. 37, pp. 5104–5110.
Wang, J., Su, S., and Qiu, J., Mrs Advances, 2016, vol. 1, no. 19, pp. 1333–1338.
Huang, Y., Gao, Y., Zhang, Q., et al., J. Hazardous Materials, 2018, vol. 354, pp. 54–62.
El-Moneim, A. A., Akiyama, E., Habazaki, H., et al., Cor. Sci., 1998, vol. 40, no. 9, pp. 1513–1531.
Marbén, G., López, I., and Valdés-Solís, T., Appl. Cat., A, General, 2009, vol. 361, no. 1, pp. 160–169.
Singh, D.K., Iyer, P.K., and Giri, P.K., Carbon, 2012, vol. 50, no. 12, pp. 4495–4505.
Jang, M.H., Song, S.H., Ha, H.D., et al., Carbon, 2017, vol. 118, pp. 524–530.
Ghiorso, M.S., Hirschmann, M.M., Reiners, P.W., et al., Geochem. Geophy. Geosys., 2013, vol. 3, no. 5, pp. 1–35.
Mourya, P., Banerjee, S., and Singh, M.M., Cor. Sci., 2014, vol. 85, pp. 352–363.
Hejazi, S., Mohajernia, S., Moayed, M.H., et al., J. Ind. & Eng. Chem., 2015, vol. 25, pp. 112–121.
Bedair, M.A., El-Sabbah, M.M.B., Fouda, A.S., et al., Corros. Sci., 2017, vol. 128, pp. 54–72.
Cui, M., Ren, S., Zhao, H., et al., Appl. Sur. Sci., 2018, vol. 443, pp. 145–156.
Abd El-Lateef, H.M., Abu-Dief, A.M., Abdel-Rahman, L.H., et al., J. Electroanal. Chem., 2015, vol. 743, pp. 120–133.
Duan, Y., Factors Determining and Limiting the Impedance Behavior of Implanted Bioelectrodes in Smart Structures & Devices, International Society for Optics and Photonics, 2001.
Heloisa, A. Acciari, Guastaldi, A. C., and Brett. C. M. A., Corros. Sci., 2005, vol. 47, no. 3, pp. 635–647.
Gonzélez-Rodríguez, C.A., Rodríguez-Gómez, F.J., and Genescé-Llongueras, J., Electrochimica Acta, 2008, vol. 54, no. 1, pp. 86–90.
Khaled, K.F. and Al-Qahtani, M.M., Materials Chemistry & Physics, 2009, vol. 113, no. 1, pp. 150–158.
Zhang, J., Wei-Zhao, Y.U., Yan, Y. G., et al., Acta Physico-Chimica Sinica, 2010, vol. 26, no. 5, pp. 595–611.
Thabo, P., Lukman, O., Indra, B., et al., Molecules, 2015, vol. 20, no. 9, pp. 16004–16029.
Pourghasemi Hanza, A., Naderi, R., Kowsari, E., et al., Corros. Sci., 2016, pp. 96–106.
Zarrouk, A., Hammouti, B., Lakhlifi, T., et al., Corros. Sci., 2015, vol. 90, pp. 572–584.
Acknowledgments
The authors gratefully acknowledged financial support and research facilities provided by the Southwest Petroleum University, Institute, Chengdu, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors state that there is no conflict of interest to be disclosed in the present communication.
Rights and permissions
About this article
Cite this article
Lv, J., Fu, L., Zeng, B. et al. Synthesis and Acidizing Corrosion Inhibition Performance of N-Doped Carbon Quantum Dots. Russ J Appl Chem 92, 848–856 (2019). https://doi.org/10.1134/S1070427219060168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427219060168