Abstract
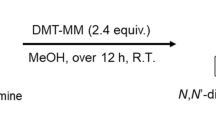
The present study focusing on design and evaluation of series of eight new structurally related dithiodiglycolamides (DTDGA) as a novel promising solvent extraction reagents. The influence of the nature of the alkyl chain on the distribution ratio of Pd(II) was investigated. Both N, N-di-hexyl-N′, N′-di-octyldithiodiglycolamide (DHDODTDGA) and N, N-di(2-ethylhexyl)-N′, N′-dioctyldithiodiglycolamide (DEHDODTDGA) were chosen and applied to perform the selective recovery and separation of Pd(II) from certain commonly associated elements such as Pt(IV), Rh(III), Fe(III), Cr(II), Mn(II), Zr(II), and Ni(II) contained in hydrochloric acid solutions using n-dodecane as diluent. A systematic investigation has been carried to understand the extraction behavior of Pd(II) using the synthesized extradant. The extraction equilibrium of Pd(II) was obtained within 3–4 min. The investigated extractants showed quantitative extraction of Pd(II) at ∼ 4 M HCl. The main extracted species of Pd(II) at 3.5 M HCl is Pd.DTDGA and IR spectra of the extracted species have been also studied. The other investigated metals ions were found poorly extracted under the same extraction contortions. Quantitative back-extraction of Pd(II) in the organic phase was obtained in single contact using thiourea solution. The obtained results make the novel synthesized ligands a promising candidates for selective recovery and separation of Pd(II) from spent catalyst dissolver (SSCD) solution.
Similar content being viewed by others
References
Kononova, O.N., Duba, E.V., Shnaider, N.I., Pozdnyakov, I.A., Russ. J. Appl. Chem., 2017, vol. 90, pp. 1239–1245.
Golubyatnikova, L.G., Mulagaleev, R.F., Khisamutdinov, R.A. et al., Russ. J. Appl. Chem., 2017, vol. 90, pp. 1475–1479.
Mowafy, E.A., and Aly, H.F., J. Hazardous Materials, 2007, vol. 149, pp. 465–470.
Ruhela, R., Sharma, J.N, Tomar, B.S., Panja, S., Tripathi, S.C., Hubli, R.C., and Suri, A.K., Radiochim Acta, 2010, vol. 98, pp. 209–214.
Mohamed, D., Russ. J. Appl. Chem., 2016, vol. 89, pp. 1322–1327.
Mowafy, E.A., Mohamed, D., and Alshammri, A., Sep. Sci. Tech., 2015, vol. pp. 50: 2352–2359.
Report on Critical Raw Materials for the EU-European Commission, May 2014. https://doi.org/ec.europa.eu/docsroom/documents/10010/attachments/1/translations/en/renditions/pdf.
Ndlovu, J., In Proceedings of the Exchange of Good Practices on Metal By-Products Recovery Technology and Policy Challenges, Brussels, Belgium, 12–13 November 2015.
Anpilogova, G.R., Khisamutdinov, R.A., Golubyatnikova, L.G., and Murinov, Yu.I., Russ. J. Appl. Chem., 2016, vol. 89, pp. 206–211.
Anpilogova, G.R., Khisamutdinov, R.A., and Murinov, Yu.I., Russ. J. Appl. Chem., 2010, vol. 83, pp. 945–950.
Steinlechner, S. and Antrekowitsch, J., JOM, 2015, vol. 67, pp. 406–411.
Men’shikov, V.I., Voronova, I.Yu., Proidakova, O.A., Malysheva, S.F., Ivanova, N.I., Belogorlova, N.A., Gusarova, N.K., and Trofimov, B.A., Russ. J. Appl. Chem., 2009, vol. 82, pp. 183–189.
Zhidkova, T.I., Belova, V.V., Brenno, Y.Y., Zhidkov, L.L., and Khol’kin, A.I., Russ. J. Inorg. Chem., 2009, vol. 54, pp. 1502–1506.
Tikhomirova, V.I., Desyatova, T.A., and Suvorova, V.A., Russ. J. Appl.Chem., 2007, vol. 80, pp. 1636–1642.
Narita, H., Tanaka, M., and Morisaku, K., Abstracts of Papers, Proceedings of the International Solvent Extraction Conference, Beijing, China, 19–23 September 2005, Beijing, 2005, pp. 227–232.
Mowafy, E.A., and Mohamed, D., Oriental J. Chem., 2017, vol. 33(5), pp. 2377–2385.
Mowafy, E.A., and Aly, H.F., Solvent Extr. Ion Exch., 2007, vol. 25, pp. 205–224.
Mowafy, E.A., and Mohamed, D., Sep. Puri. Tech., 2014, vol. 128, pp. 18–24.
Narita, H., Tanaka, M., and Morisaku, K., Min. Eng., 2008, vol. 21, pp.483–488.
Ruhela, R., Sharma, J.N., Tomar, B.S., Murali, M.S., Hubli, R.C., and Suri, A.K., Tetrahedron Lett., 2011, vol. 52, pp. 3929–3932.
Paiva, A.P., Martins, M.E., and Ortet, O., Metals, 2015, vol. 5, pp. 2303–2315.
Mowafy, E.A., and Mohamed, D., Desalination Water Treatment, 2017, vol. 68, pp. 190–198.
Das, A., Ruhela, R., Singh, R., Singh, A.K., and Hubli, R.C., Sep. Puri. Tech., 2014, vol. 125, pp. 151–155.
Mowafy, E.A., and Aly, H.F., J. Radioanal. Nucl. Chem., 2000, vol. 250(1), pp. 199–203.
Paiva, A.P., Carvalho, G.I., Costa, M.C., Costa, A.M.R., and Nogueira, C., Solvent Ext. Ion Exch., 2014, vol., 32, pp. 78–94.
Clayden, J., Greeves, N., Warren, S., and Wothers, P., Organic Chemistry, Oxford University Press, New York, 2001.
Paiva, A.P., Carvalho, G.I., Costa, M.C., Costa, A.M.R., and Nogueira, C., Solvent Ext. Ion. Exch. 2014, vol. 32, pp. 78–94.
Yamada, M., Gandhi, M.R., Sato, D., Kaneta, Y., and Kimura, N., Ind. Eng. Chem. Res., 2016, vol. 55, pp. 8914–8921.
Hagelüken, C., Platinum Metals Rev., 2012, vol. 56, pp. 29–35. https://doi.org/ec.europa.eu/docsroom/documents/10010/attachments/1/translations/en/renditions/pdf.
Singh, K.K., Ruhela, R., Das, A., Kumar, M., Singh, A.K., Hubli, R.C., and Bajaj, P.N., J. Environ. Chem. Eng., 2015, vol. 3, pp. 95–103.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mowafy, E.A., Al Shammari, A.M. Recycling of Palladium and Selected Metal Ions from Simulated Spent Catalysis Waste Solution Using Novel Dithiodiglycolamides Derivatives. Russ J Appl Chem 92, 310–320 (2019). https://doi.org/10.1134/S1070427219020228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427219020228