Abstract
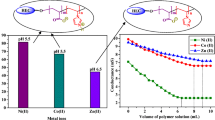
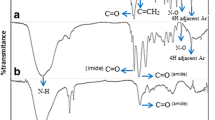
the work, 3-(4-hydroxy phenyl)cyclopropane-1,1,2,2-tetramethyleneamine (HPCA) was synthesized. Poly(styrene-maleic anhydride) (SMA) copolymer was modified with HPCA and subsequently the product (SMA–HPCA) reacted with 1,2-diaminoethane (DAE) for preparation of tridimensional chelating resin (CSMA–HPCA) as a new copolymer with multiprimary amines cyclopropane functionalities in the pendant group and applied to remove heavy metal ions such as Cu(II), Pb(II), Zn(II) from aqueous solution. The adsorption behavior of selected metal ions was investigated by synthesized resins in various experimental conditions, under change in pH, metal ion concentration, and contact time. The CSMA–HPCA showed a high tendency for removing the selected metal ions compared to SMA–HPCA and the affinity order was: Cu(II) > Zn(II) > Pb(II). Kinetics studies revealed that the adsorption process onto CSMA–HPCA followed the pseudo-second order kinetics and adsorption experimental data were well fitted to Langmuir isotherm. The synthesized resins and their metal chelates were characterized by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), gravimetry, UV- Vis spectroscopy, and atomic absorption techniques (AAS).
Similar content being viewed by others
References
Rao, M.M., Rao, G.P.C., Seshaiah, K., Choudary, N.V., and Wang, M.C., Waste Manag. 2008, vol. 28, no. 5, pp. 849–858.
Yu, B., Zhang, Y., Shukla, A., Shukla, S.S., and Dorris, K.L., J. Hazard. Mater., 2001, vol.84, no. 1, pp. 83–94.
Kumar, R., Kumar, M., Ahmad, R., and Barakart, M.A., Chem. Eng. J. 2013, vol. 218, pp. 32–38.
Liang, X., Su, Y., Yang, Y., and Qin, W., J. Hazard. Mater., 2012, vol.203, pp. 183–187.
Liu, D., Li, Z., Li, W., Zhong, Z., Xu, J., Ren, J., and Ma, Z., Ind. Eng. Chem. Res. 2013, vol. 52, no. 32, pp. 11036–11044.
Ozkula, G., Furbano, B., Rivas, B., Kabay, N., and Bryjak, M., J. Chil. Chem. Soc., 2016, vol. 61, pp. 2752–2756.
Naushad, M. and Alotman, Z.A., Desaline Water Treat J., 2015, vol. 53, pp.2158–2166.
Petersková, M., Valderrama, C., Gibert, O., and Cortina, J.L., Desalination, 2013, vol. 286, pp.316–323.
Luo, S.L., Li, X.J., Chen. L., Chen, J.L., Wan. Y., Liu, C.B., Chem. Eng. J., 2014, vol. 239, pp.312–321.
Gerente, C., Lee, V., Le Cloirec, P., McKay, G., Crit. Rev. Environ. Sci. Technol., 2007, vol. 37, no. 1, pp. 41–127.
Abdel-Galil, E.A., Sharaf El-Deen, G.E., El-Aryan, Y.F., and Khalil, M., Russ. J. Appl. Chem., 2016, vol. 89, no. 3, pp. 467–479.
Atta, A.M, Al-Lohedan, H.A, Ezzat, A.O, Issa, Z. A., and Oumi, A.B., J. Polym. Res. 2016, vol. 23, pp. 1–11.
Zhao, D., Zhang, Z., Xuan, H., Chen, Y., Zhang, K., and Alsaedi, A., J. Colloid. Interface. Sci., 2016, vol. 506, pp. 300–307.
Coskun, R., and Dilci, Y., J. Macromol. Sci., Pure Appl. Chem., 2014, vol. 51, no. 10, pp. 767–782.
Hosseinzadeh, M., Noroozi Pesyan, N., and Najafi Moghadam, P., Adv. Polym. Tech., 2018, vol. 37, no. 2, pp. 461–467.
Karimi Neghlani, P., Ra fizadeh, M., Af shar Taromi, F., J. Hazard. Mater., 2011, vol. 186, no. 1, pp. 182–189.
Mohammadnezhad, G.H., Soltani, R., Abad, S., and Dinari, M., J. Appl. Polym. Sci., 2017, vol. 134, no. 40, p. 45383.
Elwakeel, K.Z., and Al-Bogami, A.S., J. Hazard. Mater., 2018, vol. 342, pp. 335–346.
Hasanzadeh, R., Najafi Moghadam, P., and Samadi, N., Polym. Adv. Technol., 2013,vol. 24, pp.34–41.
Abo-Baker, E., Elkholy, Said S., and Elsabee, Maher Z., Am. J.Polym. Sci. 2015, vol. 5, no. 3, pp. 55–64.
Najafi Moghadam, P., Hasanzadeh, R., and Khalafy, J., Iran. Polym. J. 2013, vol. 22, pp. 133–142.
Masoumi, A., and Ghaemy, M., Express. Polym. Lett. 2014, vol. 8, pp. 187–196.
Samadi, N., Ansari, R., and Khodavirdilo, B., Am. J. Phytomedicine & Clinical Therapeutics, 2015, vol. 3, pp. 451–468.
Hartman, W.W. and Dreger, E.E., Org. Synth. Coll. 1943, vol. 2, pp. 150–151.
Henry, S.M., El-Sayed, M.E.H., Pirie, C.R.M., Hoffman, A.S., and Stayton, P.S., Biomacro. Molecules 2006, vol. 7, pp. 2407–2414.
Wang, C-C., Chang, C-Y., and Chen, C-Y., Macromol. Chem. Phys. 2001, vol. 202, pp. 882–890
Mahmoud, M.E., Osman, M.M., Hafez, O.F., Hegazi, A.H., Elmelegy, E., Desalination, 2010, vol. 251, no. 1–3, pp. 123–130.
Switala, Z.M., Polym. Degrad. Stable 2006, vol. 91, no. 5, pp. 1233–1239.
Chen, C.L., Wang, X.K., and Nagatsu, M., Environ. Sci. Technol. 2009, vol. 43, pp. 2362–2367.
Chen, C.L., Hu, J., Shao, D.D., Li, J.X., and Wang, X.K., J.Hazard. Mater., 2009, vol. 164, pp. 923–928.
Gomes, E.C.C., de Sousa, A.F., Vasconcelos, P.H.M., Melo, D.Q., Diógenes, I.C.N., and de Sousa, E.H.C., Chem. Eng. J. 2013, vol. 214, pp. 27–33.
Zhao, G.X., Li, J.X., Ren, X.M., Chen, C.L., and Wang, X.K., Environ. Sci. Technol. 2011, vol. 45, pp. 10454–10462.
Huang, Y.X. and Keller, A.A., Water Res. 2015, vol. 80, pp. 159–168.
Jeong, J.H., Byoun, Y.S., Ko, S.B., and Lee, Y.S., J.Ind. Eng. Chem., 2001, vol. 7, pp. 310–315.
Samadi, N., Ansari R., and Khodavirdilo, B., Egypt. J. Pet., 2017, vol. 26, pp. 375–389.
Syed Ibrahima, G.P., Isloor, A.M., Asirib, A.M., Ahamed, M.I., Chem. Eng. J. 2018, vol. 353, pp. 425–435.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the author in English.
Rights and permissions
About this article
Cite this article
Hosseinzadeh, M. Removal of Heavy Metal Ions from Aqueous Solutions Using Modified Poly(styrene-alt-maleic anhydride) Copolymer as a Chelating Resin. Russ J Appl Chem 91, 1984–1993 (2018). https://doi.org/10.1134/S1070427218120108
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427218120108