Abstract
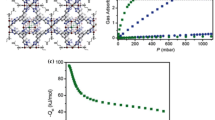
A series of porous coordination polymers of Zn-oxac-ATaz (oxac: oxalic acid and ATaz: 3-amino,1,2,4- triazole), Zn-oxac-Taz (Taz: 1,2,4-triazole), and combination of ATaz–Taz ligand with 0.5 molar ratio X of Zn-oxac-Taz/ATaz were synthesized under magnetic fields. There is no even significant XRD pattern change of Zn-oxac-Taz 6T but there are noticeable changes in the morphology of irregular agglomerates at zero field to rectangular-prism crystals with smooth surfaces. Compared to the zero field, it is obvious that magnetic fields also bring significance morphology and crystal orientation change of Zn-oxac-ATaz 4T and Zn-oxac-Taz/ATaz 4T with growth to prolong rectangular morphology. Zn-oxac-Taz/ATaz 0T adsorbed more CO2 (135 mg g–1) at 303 K after heating in a vacuum at 333K for 12 h. It is suggested that the integration effects of pore space and amine group presences inside the frameworks induce to enhance carbon dioxide adsorption amount. Furthermore, due to magnetic fields the CO2 adsorption by Zn-oxac-Taz/ATaz 4T also increases to 155.46 mg g–1 and Langmuir surface areas developed is 467 m2 g–1. Magnetic fields cause interesting phenomena observed in XRD pattern and through morphological changes inducing the enhancement carbon dioxide (CO2) capture in these porous coordination series.
Similar content being viewed by others
References
Kitagawa, S., Kitaura, R., and Noro, S., Angew. Chem. Int. Ed, 2014, no. 43, pp. 2334–2375.
Shimomura, S., Horike, S., Matsuda, R., and Kitagawa, S., J. Am. Chem. Soc, 2007, vol. 129 pp. 10990–10991.
Li, H., Eddaoudi, M., Groy, T.L., and Yaghi, O.M., J. Am. Chem. Soc, 1998, pp. 8571–8572.
Li, W., Jia, H.P., Ju, Z., and Zhang, J.A., Crystal Growth & Design, 2006, vol. 6, no. 9, pp. 2136–2140.
Garcia-Ricard, O.J., Morales, P.M., Martinez, J.C.S., Curet-Arana, M.C.J., Hogan, A., Hernandez-Maldonado, A.J., Microporous and Mesoporous Materials, 2013, vol. 177, pp. 54–58.
Noro, S., Kitagawa, S., Akutagawa, T., Nakamura, T., Prog. Polym. Sci., 2009, vol. 34, pp. 240–279.
Millward, A.R. and Yaghi, O.M., J. Am. Chem. Soc., 2005, vol. 127, pp. 17998–17999.
Li, H.L. Eddaoudi, M., O’Keeffe, M., and Yaghi, O.M., Nature, 1999, vol. 402, pp. 276–279.
Yang, Q.Y., Xue, C.Y., Zhong, C.L., and Chen, J.F., AICHE J., 2007, vol. 53, pp. 2832–2840.
Arstad, B., Fjellvag, H., Kongshaug, K.O., Swang, O., and Blom, R., Adsorption, 2008, vol. 14 pp. 755–762.
Vaidhyanathan, R., Iremonger, S.S., Dawson, K.W., and Shimizu, G.K.H., Chem. Commun, 2009, pp. 5230–5232.
Ozeki, S., Kurashima, H., and Abe, H., J. Phys. Chem. B., 2000, vol. 104, pp. 5657–5660.
Yamaguchi, M. and Tanimoto, Y., Magneto-Science, Springer, Tokyo, 2006.
Saravanan, G. and Ozeki, S., J. Phys. Chem. B., 2008, vol. 112, pp. 3–6.
Ozeki, S. and Otsuka, I., J. Phys. Chem. B., 2006, vol.110, pp. 20067–20072.
Otsuka, I. and Ozeki, S., J. Phys. Chem. B., 2006, vol.110, pp. 1509–1512.
Zubir, M., Hamasaki, A., Ohta, A., Ohki, H., and Ozeki, S., Langmuir, 2017, vol. 33 pp. 680–684.
Zubir, M., Hamasaki, A., Iiyama, T., Ohta, A., Ohki, H., and Ozeki, S., Chem. Lett., 2016, vol. 45, pp. 362–364.
Deng, H.X., Doonan, C.J., Furukawa, H., Ferreira, R.B., Towne, J., Knobler, C.B., Wang, B., and Yaghi, O.M., Science, 2010, vol. 327, pp. 846–850.
Altomare, A., Cuocci, C., Giacovazzo, C., Moliterni, A., Rizzi, R., Corriero, N., and Falcicchio, A., Appl. Cryst., 2013, vol. 46, pp. 1231–1235.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Zubir, M., Nasution, H.I. & Sudarma, T.F. Synthesis of Porous Coordination Polymers Comprising Mixed Ligands of Triazole and Amino Triazole under Magnetic Fields and Its Effects in Enhance CO2 Adsorptivity. Russ J Appl Chem 91, 1867–1873 (2018). https://doi.org/10.1134/S1070427218110186
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427218110186