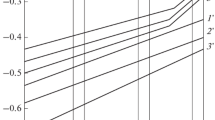
Abstract
Influence exerted by the nature and concentration of an electrolyte and by the electrolysis temperature on the process of anodic oxidation of aluminum in aqueous solutions was studied. It was shown that two modifications of aluminum hydroxide are formed as electrolysis products. Making higher the electrolyte concentration and raising the process temperature results in that the fraction of boehmite aluminum hydroxide in the product increases and that of bayerite aluminum hydroxide decreases. A scheme by which aluminum is anodically oxidized in aqueous solutions is suggested.
Similar content being viewed by others
References
Mirzoev, R.A. and Davydov, A.D., Anodnye protsessy elektrokhimicheskoi i khimicheskoi obrabotki metallov (Anodic Processes in Electrochemical and Chemical Processing of Metals), St. Petersburg Politekhn. Univ., 2013.
Ponomarev, A.F. and Stanislavchik, V.F., Elektron. Tekh., Ser. Radiodet. Radiokomp., 1984, no. 3, pp. 13–15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © R.K. Bairamov, D.Z. Ishmukhametov, Yu.Yu. Somova, 2017, published in Zhurnal Prikladnoi Khimii, 2017, Vol. 90, No. 3, pp. 286−291.
Rights and permissions
About this article
Cite this article
Bairamov, R.K., Ishmukhametov, D.Z. & Somova, Y.Y. Behavior of aluminum in its anodic oxidation in aqueous solutions of acids and their salts. Russ J Appl Chem 90, 349–353 (2017). https://doi.org/10.1134/S1070427217030041
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427217030041