Abstract
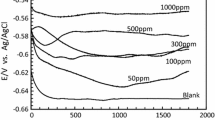
In this research, we first prepared poly (naphthylamine-formaldehyde) (PNAF) and then investigated its corrosion inhibition properties for polished steel specimens in 1 M HCl solution using chemical and electrochemical methods. Results showed that the PNAF could serve as an effective inhibitor of the corrosion of steel in hydrochloric acid media (the inhibition efficiency of this polymer at an optimum concentration of 100 mg L–1 was 99.9 %). The effect of temperatures on the corrosion behavior of steel was studied in the temperature ranging from 303 to 333 K for 1 M HCl at an optimum concentration of PNAF. It has been demonstrated that the adsorption behavior of this polymer on steel in 1 M HCl was found to obey Langmuir adsorption isotherm. Also, potentiodynamic polarization measurements showed that PNAF was a mixed type inhibitor.
Similar content being viewed by others
References
Ahmad, N. and MacDiarmd, A.G., Synth. Met., 1996, vol. 78, p. 103.
Subasri, R., Shinohara, T., and Mori, K., Sci. Technol. Adv. Mater., 2005, vol. 6, p. 501.
Kim, D.K., Muralidharan, S., Ha, T.H., Bae, J.H., Ha, Y.C. and Lee, H.G., Electrochim. Acta, 2006, vol. 51, p. 5259.
Cecchetto, L., Delabouglise, D., and Petit, P., Electrochim. Acta, 2007, vol. 52, p. 3485.
Praveen, B.M., Venkatesha, T.V., Arthoba Naik, Y., and Prashantha, K., Surf. Coating Technol., 2007, vol. 201, p. 5836.
Lagrenee, M., Mernari, B., Bouanis, M., Traisnel, M., and Bentiss, F., Corros. Sci., 2002, vol. 44, p. 573.
Quraishi, M.A. and Sardar, R., Corrosion, 2002, vol. 58, p. 748.
Quraishi, M.A., Athar, M., and Ali, H., Br._Corrosion J., 2002, vol. 37, p. 155.
Quraishi, M.A. and Ansari, F.A., J. Appl. Electrochem., 2003, vol. 33, p. 233.
Quraishi, M.A. and Khan, S., J. Appl. Electrochem., 2006, vol. 36, p. 539.
Quraishi, M.A., Ahmad, I., Singh, A.K., Shukla, S.K., Lal, B., Singh, V., Mater. Chem. Phys., 2008, vol. 112, p. 1035.
Bentiss, F., Lagrenee, M., Traisnel, M., and Hornez, J.C., Corros. Sci., 1999, vol. 41, p. 789.
Growcock, F.B., Frenier, W.W., and Andreozzi, P.A., Corrosion, 1989, vol. 45, p. 1007.
Lukovits, I., Kalman, E., and Palinkas, G., Corrosion, 1995, vol. 51, p. 201.
Robert, C., Ayers, Jr., and Hackerman, N., J. Electrochem. Soc., 1963, vol. 110, p. 507.
Shukla, S.K., Quraishi, M.A., and Prakash, R., Corros. Sci., 2008, vol. 50, p. 2867.
Quraishi, M.A. and Shukla, S.K., Mater. Chem. Phys., 2009, vol. 113, p. 685.
Rajendran, S., Sridevi, S.P., Anthony, N., Amalraj, A.J., and Sundearavadivelu, M., Anti-Corrossion Methods Mater., 2005, vol. 52, p. 102.
Grchev, T., Cvetkovska, M., and Schultze, J.W., Corros. Sci., 1991, vol. 32, p. 103.
Manivel, P. and Venkatachari, G., J. Appl. Polym. Sci., 2007, vol. 104, p. 2595.
Manivel, P. and Venkatachari, G., J. Met. Mater. Sci., 2004, vol. 46, p. 165.
Manivel, P. and Venkatachari, G., J. Mater. Sci. Technol., 2006, vol. 22, p. 301.
Sathiyanarayanan, S., Dhawan, S.K., Trivedi, D.C. and Balakrishnan, K., Corros. Sci., 1992, vol. 33, p. 1831.
Siddaraman, 1st ed., CBS Publishers Bangalore, 2005, p. 43.
Schorr, M. and Yahalom, J., Corros. Sci., 1972, vol. 12, p. 867.
Quraishi, M.A., Singh, A., Singh, V.K., Yadav, D.K. and Singh, A.K., Mater. Chem. Phys., 2010, vol. 122, p. 114.
Ansari, K.R. and Quraishi, M.A., J. Ind. Eng. Chem., 2014, vol. 20, p. 2819.
Hussin, M.H. and Kassim, M.J., Mater. Chem. Phys., 2011, vol. 25, p. 461.
Morad, M.S. and Kamal El-Dean, A.M., Corros. Sci., 2006, vol. 48, p. 3398.
Sahin, M., Bilgic, S., and Yilmaz, H., Appl. Surf. Sci., 2002, vol. 195, p. 1.
Ateya, B., El-Anadouli, B.E., and El-Nizamy, F.M., Corros. Sci., 1984, vol. 24, p. 509.
Li, X.H., Deng, S.D., Mu, G.N., Fu, H., and Yang, F.Z., Corros. Sci., 2008, vol. 50, p. 420.
Badr, G.E., Corros. Sci., 2009, vol. 51, p. 2529.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Norouzi, B., Yousefi, J. & Nami, N. Preparation of poly(naphthylamine-formaldehyde); Application as a new and an effective inhibitor of steel in hydrochloride acid solution. Russ J Appl Chem 89, 1879–1886 (2016). https://doi.org/10.1134/S1070427216110203
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427216110203