Abstract
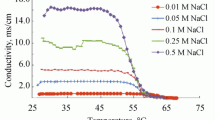
Nanoemulsions are transparent or semi-transparent systems mostly covering the size range of 50–200 nm. In the research, the production of nanoemulsion of n-dodecane in water was studied by phase inversion temperature method (PIT), and the effect of the changes in NaCl and surfactant concentration on PIT and droplet size of n-dodecane was studied. The changes in conductivity with temperature were measured for emulsions containing 20 wt % dodecane, 4 and 8 wt % Brij30, and NaCl of various concentrations in aqueous phase. The results showed a decrease in PIT with a rise in concentration of NaCl and also Brij30. There was a linear model fitted to the data. Analysis of variance (ANOVA) shows that, the significance value (p-value) is less than 0.05, and the effect of the changes in concentration of surfactant Brij30 on the decrease in PIT and droplet size was the more than that in concentration of NaCl in aqueous phase. The lowest mean droplet diameters were in the range of 8.6–14 nm.
Similar content being viewed by others
References
Tadros, Th.F., Izquierdo, P., Esquena, J., and Solans, C., Adv. Colloid Interface Science, 2004, vol. 108, pp. 303–318.
Tadros, Th. F., Vandamme, A., Levecke, B., Booten, K., and Stevens, C.V., Adv. Colloid. Interf. Sci., 2004, vol. 108, 109, pp. 207–226.
Bouchemal, K., Briançon, S., Perrier, E., and Fessi, H., Int. J. Pharm., 2004, vol. 280, nos. 1, 2, pp. 241–251.
Solans, C., Izquierdo, P., Nolla, J., Azemar, N., and Garcia-Celma, M.J., Current Opinion in Colloid & Interface Sci., 2005, vol. 10, nos. 3, 4, pp. 102–110.
Solans, C., Esquena, J., Forgiarini, A.M., Ulson, N., Morales, D., Izquierdo, P., Azemar, N., and Garcia-Celma, M.J., Absorption and Aggregation of Surfactants in Solution, vol. 109, New York: Marcel Dekker, 2003.
Fernandez, P., Andrè, V., Rieger, J., and Kühnle, A., Colloids & Sur. A: Physicochem., Eng. Aspects, 2004, vol. 251, no. 1–3, pp. 53–68.
Morales, D., Gutiérrez, J.M., García-Celma, M.J., and Solans, C., Langmuir, 2003, vol. 19, no.18, pp. 7196–7200.
Shinoda, K. and Saito, H.J., Colloid Interface Sci., 1969, vol. 30, no. 2, pp. 258–263.
Engels, T., Forster, W., and Rybinski, von W., Colloid. Surf. A, 1995, vol. 99, no. 2, 3, pp. 141–149.
Lin Ee, Sh., Duan, Xi., Liew, Jef., and Dzuy Nguyen, Q., Chem. Eng. J., 2008, vol. 140, nos. 1–3, pp. 626–631.
Salager, J.L., Encyclopedia of Emulsion Technology, vol. 3, New York: Marcel Dekker, 1988.
Salager, J.L., Marquez, L., Mira, I., Pena, A., Tyrode, E., and Zambrano, N.B., Absorption and Aggregation of Surfactants in Solution, vol. 109, New York: Marcel Dekker, 2003.
Brooks, B.W., Richmond, H.N., and Zerfa, M., Modern Aspects of Emulsion Science, Cambridge, 1998.
Bouchama, F., van Aken, G.A., Autin, A.J.E., and Koper, G.J.M., Colloid Surf. A, 2003, vol. 231, nos. 1–3, pp. 11–17.
Taisne, L. and Cabane, B., Langmuir, 1998, vol. 14, no. 17, pp. 4744–4752.
Astaraki, A.M., Mehrdad Sharif, A.A., Abroomand Azar, P., Abedini Khorrami, S., and Moradi, Sh., Int. J. Acad. Res., 2010, vol. 2, no. 6, pp. 114–119.
Izquierdo, P., Esquena, J., Tadros, Th.F., Dederen, C., Garcia, M.J., Azemar, N., and Solans, C., Langmuir, 2002, vol. 18, no. 1, pp. 26–30.
Mehrdad Sharif, A.A., Astaraki, A.M., Aberoomand Azar, P., Abedini Khorrami, S., and Moradi, Sh., Arab. J. Chem., 2012, vol. 5, no. 1, pp. 41–44.
Ghosh, V., Mukherjee, A., and Chandrasekaran, N., Ultrasonics Sonochem., 2013, vol. 20, no. 1, pp. 338–344.
Schalbart, P., Kawaji, M., and Fumoto, K., Int. J. Refrigeration, 2010, vol. 33, no. 8, pp. 1612–1624.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the author in English.
Rights and permissions
About this article
Cite this article
Astaraki, A.M. The effect of concentration of surfactant and electrolyte on the pit and droplet sizes nanoemulsions of n-dodecane in water. Russ J Appl Chem 89, 84–89 (2016). https://doi.org/10.1134/S10704272160010134
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S10704272160010134