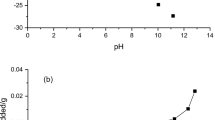
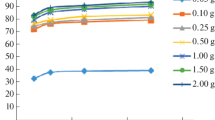
Abstract
Effect of various complexons [ethylenediaminetetraacetic acid, nitrilotriacetic acid, iminodiacetic acid, ethylenediaminetetra(methylenephosphonic acid), nitrilotris(methylenephosphonic acid)] on the sorption of heavy metal cations Cu2+, Ni2+, Pb2+, Zn2+ by crystalline and amorphous Fe(III), Al(III), and Mn(IV) oxides and hydroxides was studied. The sorption processes in systems constituted by a metal cation, complexon, and sediment were analyzed in terms of the theory of surface complexation. The factors affecting the processes of remobilization/immobilization of heavy metals under the action of complexons were studied. The desorbing effect of complexons becomes stronger with increasing stability and decreasing sorption capacity of complexonates formed in solution. A flow chart of recovery of heavy metals from contaminated sediments with ethylenediaminetetraacetic acid was developed.
Similar content being viewed by others
References
Drugov, Yu.S. and Rodin, A.A., Analiz zagryaznennoi pochvy i opasnykh otkhodov (Analysis of Contaminated Soil and Hazardous Wastes), Moscow: BINOM, Laboratoriya Znanii, 2013.
Vodyanitskii, Y.N., Eurasian Soil Sci., 2012, vol. 45, no. 3, pp. 321–328.
Dermont, G., Bergeron, M., Mercier, G., and RicherLaflche, M., J. Hazard. Mater., 2008, vol. 152, no. 1, pp. 1–31.
Leštan, D., Luo Chun-ling, and Li Xiang-dong, Environ. Pollut., 2008, vol. 153, no. 1, pp. 3–13.
Knepper, T.P., Trends Anal. Chem., 2003, vol. 22, no. 19, pp. 708–724.
The Environmental Chemistry of Aluminum, Sposito, G., Ed., CRC Press, 1996.
Cornell, R.M. and Schwertmann, U., The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, Wiley-VCH, 2003.
Sparks, D.L., Environmental Soil Chemistry, Acad. Press, 2003.
NIST Standard Reference Database 46: NIST Critically Selected Stability Constants of Metal Complexes: Version 8.0. http://www.nist.gov/srd/nist46.cfm
Tonkin, J.W., Balistrieri, L.S., and Murray, J.W., Appl. Geochem., 2004, vol. 19, no. 1, pp. 29–53.
Cristiano, E., Hu, Y., Siegfried, M., et al., Clay Clay Miner., 2011, vol. 59, no. 2, pp. 107–115.
Nowack, B. and Stone, A.T., Environ. Sci. Technol., 1999, vol. 33, no. 20, pp. 3627–3633.
Zenobi, M.C. and Rueda, E.H., Quím. Nova, 2012, vol. 35, no. 3, pp. 505–509.
Kropacheva, T.N., Didik, M.V., and Kornev, V.I., Sorbts. Khromatogr. Protsessy, 2013, vol. 13, no. 3, pp. 360–368.
Alkatsen, M.I., Protsessy tsementatsii v tsvetnoi metallurgii (Cementation Processes in Nonferrous Metallurgy), Moscow: Metallurgiya, 1981.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.N. Kropacheva, A.S. Antonova, Yu.V. Rabinovich, V.I. Kornev, 2014, published in Zhurnal Prikladnoi Khimii, 2014, Vol. 87, No. 10, pp. 1421–1428.
Rights and permissions
About this article
Cite this article
Kropacheva, T.N., Antonova, A.S., Rabinovich, Y.V. et al. Complexons as reagents for demetallization of contaminated sediments. Russ J Appl Chem 87, 1422–1429 (2014). https://doi.org/10.1134/S107042721410005X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042721410005X