Abstract
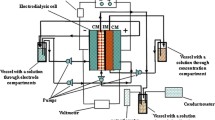
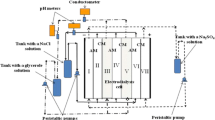
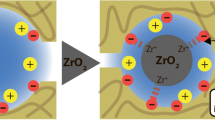
Application of inorganic composite membranes in electrodialytic desaltination of dilute solutions containing Na+, Ca2+, Mg2+, Cl−, and SO 2−4 ions was studied. The membranes are based on an oxide ceramic containing hydrated zirconium(IV) oxide as an ion-exchange component. The limiting currents were estimated theoretically.
Similar content being viewed by others
References
Koryta, J., Dvorzhak, J., and Bogachkova, V., Electrochemistry (Translated from Czech), London, 1970.
Zabolotskii, V.I. and Nikonenko, V.V., Perenos ionov v membranakh (Transfer of Ions in Membranes), Moscow: Nauka, 1996.
Ortiz, J.M., Exposito, E., Gallud F., et al., J. Membr. Sci., 2006, vol. 274, no. 2, pp. 138–149.
Dzyaz’ko, Yu.S. and Atamanyuk, V.J., Naucnye Zapiski NAUKMA, Khim. Nauki i Tekhnologii, 2004, vol. 28, pp. 50–62.
Lee, H.J., Moon, S.H., and Tsai, S.P., Separ. Purif. Technol., 2002, vol. 27, no. 2, pp. 89–95.
Lindstrand, V., Jonsson, A.S., and Sundstrom, G., Desalination, 2000, vol. 130, no. 1, pp. 73–84.
Linkov, V.N. and Belyakov, V.N., Separ. Purif. Technol., 2001, vol. 25, no. 1–3, pp. 57–63.
Amphlett, C.B., Inorganic Ion Exchangers, Amsterdam: Elsevier, 1964.
Sukharev, Yu.I., Sintez i primenenie spetsificheskikh oksigidratnykh sorbentov (Synthesis and Application of Specific Oxyhydrate Sorbents), Moscow: Energoatomizdat, 1987.
Walsh, F., A First Course in Electrochemical Engineering, London: Alresford, 1993.
Vetter, K.J., Electrochemische Kinetik, Berlin: Springer, 1961.
Ciborowski, J., Podstawy Inzynierii Chemicznej, Warsaw: Naukovo-Techniczne, 1965.
Dzyaz’ko, Yu.S., Belyakov, V.N., Stefanyak, N.V., and Vasilyuk, S.L., Zh. Prikl. Khim., 2006, vol. 79, no. 5, pp. 778–782.
Dzyaz’ko, Yu.S., Rozhdestvenskaya, L.M., and Pal’chik, A.V., Zh. Prikl. Khim., 2005, vol. 78, no. 3, pp. 418–424.
Dzyazko, Yu.S. and Belyakov, V.N., Desalination, 2004, vol. 162, pp. 179–189.
Parsons, R., Handbook of Electrochemical Constants, London: Butterworth Sci., 1959.
Balavadze, E.G., Bobreshova, O.V., and Kulintsov, P.I., Usp. Khim., 1988, vol. 57, no. 6, pp. 1031–1041.
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.M. Linkov, Yu.S. Dzyaz’ko, V.N. Belyakov, V.Yu. Atamanyuk, 2007, published in Zhurnal Prikladnoi Khimii, 2007, Vol. 80, No. 4, pp. 590–595.
Rights and permissions
About this article
Cite this article
Linkov, V.M., Dzyaz’ko, Y.S., Belyakov, V.N. et al. Inorganic composite membranes for electrodialytic desaltination. Russ J Appl Chem 80, 576–581 (2007). https://doi.org/10.1134/S1070427207040118
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070427207040118