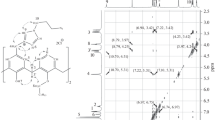
Abstract
New water-soluble derivatives of thiacalix[4]arene, p-tert-butylcalix[4]arene, and p-tert-butylthiacalix[4]arene with sulfonatopropyl- and sulfonatobutyl moieties on the lower rim were synthesized. The template effect of alkali metal cations (Na+, K+, Cs+) on the configuration of products formed in the alkylation reaction of lower rim with 1,3-propane- and 1,4-butanesultone was revealed. In the case of Na+, the cone stereoisomer was formed exclusively, while with K+ and Cs+, mixtures of stereoisomers (cone, partial cone, and 1,3-alternate) were obtained.

Similar content being viewed by others

REFERENCES
Calixarenes 2001, Asfari, M.Z., Böhmer, V., Harrowfield, J., and Vicens J., Eds., New York: Kluwer, 2007.
Calixarenes and Beyond, Neri, P., Sessler, J.L., and Wang, M.X., Eds., Basel: Springer, 2016.
Crowley, P.B., Acc. Chem. Res., 2022, p. 13764. https://doi.org/10.1021/acs.accounts.2c00206
Cation Binding by Macrocycles, Inou, Y. and Gokel, G.W., Eds., New York: Marcel Dekker, 1990.
Flood, R.J., Ramberg, K.O., Mengel, D.B., Guagnini, F., and Crowley, P.B., Cryst. Growth Des., 2022, vol. 22, no. 5, p. 3271. https://doi.org/10.1021/acs.cgd.2c00108
Gawhale, S.T., Rathod, N.V., Kalyani, V.S., Singh, P.K., Sapkal, R.S., Sapkal, V.S., Chaudhari, G.N., and Malkhede, D.D., Polyhedron, 2020, vol. 177, p. 114268. https://doi.org/10.1016/j.poly.2019.114268
Rodik, R.V., Klymchenko, A.S., Mely, Y., and Kalchenko, V.I., J. Incl. Phenom. Macrocycl. Chem., 2014, vol. 80, no. 3, p. 189. https://doi.org/10.1007/s10847-014-0412-8
García-Rio, L. and Basílio, N., Curr. Opin. Colloid Interface Sci., 2019, vol. 44, p. 225. https://doi.org/10.1016/j.cocis.2019.11.004
Kumar, R., Lee, Y.O., Bhalla, V., Kumar, M., and Kim, J.S., Chem. Soc. Rev., 2014, vol. 43, no. 13, p. 4824. https://doi.org/10.1039/C4CS00068D
Puplampu, J.B., Yakimova, L.S., Vavilova, A.A., Rizvanov, I.Kh., and Stoikov, I.I., Macroheterocycles, 2014, vol. 7, no. 4, p. 337. https://doi.org/10.6060/mhc140722s
Muravev, A., Yakupov, A., Gerasimova, T., Islamov, D., Lazarenko, V., Shokurov, A., Ovsyannikov, A., Dorovatovskii, P., Zubavichus, Y., Naumkin, A., Selektor, S., Solovieva, S., and Antipin, I., Int. J. Mol. Sci., 2022, vol. 23, no. 4, p. 2341. https://doi.org/10.3390/ijms23042341
Yakimova, L., Kunafina, A., Mostovaya, O., Padnya, P., Mukhametzyanov, T., Voloshina, A., Petrov, K., Boldyrev, A., and Stoikov, I., Int. J. Mol. Sci., 2022, vol. 23, no. 17, p. 10040. https://doi.org/10.3390/ijms231710040
Zhou, Y., Li, H., and Yang, Y.W., Chin. Chem. Lett., 2015, vol. 26, no. 7, p. 825. https://doi.org/10.1016/j.cclet.2015.01.038
Yakimova, L.S., Puplampu, J.B., Evtugin, G.A., and Stoikov, I.I., Russ. Chem. Bull., 2017, vol. 66, no. 8, p. 1515. https://doi.org/10.1007/s11172-017-1917-2
Iwamoto, K. and Shinkai, S., J. Org. Chem., 1992, vol. 57, no. 26, p. 7066-7073. https://doi.org/10.1021/jo00052a016
Iwamoto, K., Araki, K., and Shinkai, S., J. Org. Chem., 1991, vol. 56, no. 16, p. 4955. https://doi.org/10.1021/jo00016a027
Morohashi, N., Narumi, F., Iki, N., Hattori, T., and Miyano, S., Chem. Rev., 2006, vol. 106, p. 5291. https://doi.org/10.1021/cr050565j
Iki, N., Narumi, F., Fujimoto, T., Morohashi, N., and Miyano, S., J. Chem. Soc., Perkin Trans. 2, 1998, no. 12, p. 2745. https://doi.org/10.1039/A803734E
Shinkai, S., Kawabata, H., Arimura, T., Matsuda, T., Satoh, H., and Manabe, O., J. Chem. Soc., Perkin Trans. 1, 1989, no. 5, p. 1073. https://doi.org/10.1039/P19890001073
Kaddouri, M., Bouklah, M., Rekkab, S., Touzani, R., Al-Deyab, S.S., Hammouti, B., Aouniti, A., and Kabouche, Z., Int. J. Electrochem. Soc., 2012, vol. 7, no. 9, p. 9004.
Yakimova, L.S., Gilmanova, L.H., Evtugyn, V.G., Osin, Y.N., and Stoikov, I.I., J. Nanopart. Res., 2017, vol. 19, no. 5, p. 1. https://doi.org/10.1007/s11051-017-3868-9
Tauran, Y., Brioude, A., Shahgaldian, P., Cumbo, A., Kim, B., Perret, F., Coleman, A.W., and Montasser, I., Chem. Commun., 2012, vol. 48, no. 76, p. 9483. https://doi.org/10.1039/C2CC34670B
Barbera, L., Gattuso, G., Kohnke, F.H., Notti, A., Pappalardo, S., Parisi, M.F., Pisagatti, I., Patanè, S., Micali, N., and Villari, V., Org. Biomol. Chem., 2015, vol. 13, no. 23, p. 6468. https://doi.org/10.1039/c5ob00703h
Guo, D.S., Wang, K., Wang, Y.X., and Liu, Y., J. Am. Chem. Soc., 2012, vol. 134, no. 24, p. 10244. https://doi.org/10.1021/ja303280r
Sayin, S. and Yilmaz, M., Tetrahedron, 2016, vol. 72, no. 41, p. 6528. https://doi.org/10.1016/j.tet.2016.08.066
Steinmetz, A., in Catalysts Cesium from Acros Organics, Acros Organics, 2011, p. 3.
Lide, D.R., CRC Handbook of Chemistry and Physics, Boca Raton: CRC, 2004, vol. 85, p. 2661.
Cella, J.A. and Bacon, S.W., J. Org. Chem., 1984, vol. 49, no. 6, p. 1122. https://doi.org/10.1021/jo00180a033
Noll, N. and Würthner, F., Chem. Eur. J., 2021, vol. 27, no. 1, p. 444. https://doi.org/10.1002/chem.202004486
Gutsche, C.D., Iqbal, M., and Stewart, D., J. Org. Chem., 1986, vol. 51, no. 5, p. 742. https://doi.org/10.1021/jo00355a033
Iki, N., Kabuto, C., Fukushima, T., Kumagai, H., Takeya, H., Miyanari, S., Miyashi, T., and Miyano, S., Tetrahedron, 2000, vol. 56, no. 11, p. 1437. https://doi.org/10.1016/S0040-4020(00)00030-2
Funding
The work was financially supported by the Russian Science Foundation (project no. 18-73-10094, https://rscf.ru/project/18-73-10094/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
To the 145th anniversary of A.E. Arbuzov
Supplementary information
Rights and permissions
About this article
Cite this article
Nugmanova, А.R., Yakimova, L.S., Shibaeva, K.S. et al. Metal (Na+, K+, Cs+) Template Effect–Controlled Synthesis of Stereoisomers of Tetrasubstituted (Thia)calix[4]arene Derivatives Containing Sulfonatoalkyl Moieties on the Lower Rim. Russ J Gen Chem 92, 2582–2589 (2022). https://doi.org/10.1134/S1070363222120052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222120052