Abstract
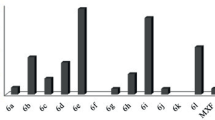
A series of novel fluoroquinolone thiazolidinone derivatives were synthesized and evaluated for their biological activity. All the newly synthesized compounds were characterized by IR, 1H NMR, 13C NMR, mass spectral techniques, and elemental analysis. Our results reveal that compounds 6a–6j have considerable activity against Gram–positive microorganisms with MICs range 0.65–64.2 μg/mL and Gram–negative strains belongs to MICs range 3.1–84.7 μg/mL respectively. From screening antibacterial results 6f, 6g, and 6d showed outstanding antibacterial activity against S. aureus with MICs 0.65, 2.2 and 4.7 µg/mL respectively whereas 6f, 6e, 6c have good potency in inhibiting the growth of P. aeruginosa including zone of inhibition 33, 32, 30 mm. The most active ligand 6d reveals highest hydrophobic binding modes with IleA:97 [2.189 Å], IleA:126 [2.199 Å], carbon hydrogen and halogen bondings with ProA:214, GluA:96, AsnA:91, Π–Π, and Π-alkyl interaction PheA:474 [2.903 Å] respectively. Compounds 6b, 6d, 6g possesses highest drug likeness model score 1.52, 1.25, 1.22, and considering their bioactivity potentials, perhaps highly substitute thiazolidinone functionalized fluoroquinolones could be the future antibiotics.



Similar content being viewed by others
REFERENCES
Wohlkonig, A., Chan, P.F., Fosberry, A.P., Homes, P., Huang, J., Kranz, M., Leydon, V.R., Miles, T.J., Pearson, N.D., Perera, R.L., Shillings, A.J., Gwynn, M.N., and Bax, B.D., Nat. Struct. Mol. Biol., 2010, vol. 17, p. 1152. https://doi.org/10.1038/nsmb.1892
Mustaev, A., Malik, M., Zhao, X., Kurepina, N., Luan, G., Oppegard, L.M., Hiasa, H., Marks, K.R., Kerns, R.J., Berger, J.M., and Drlica, K., J. Biol. Chem., 2014, vol. 289, p. 12300. https://doi.org/10.1074/jbc.M113.529164
Malik, M., Mustaev, A., Schwanz, H.A., Luan, G., Shah, N., Oppegard, L.M., de Souza, E.C., Hiasa, H., Zhao, X., Kerns, R.J., and Drlica, K., Nucleic Acids Res., 2016, vol. 44, p. 3304. https://doi.org/10.1093/nar/gkw161
Joanna, F., and Jarosław, S., Monatsh Chem., 2018, vol. 149, p. 1199. https://doi.org/10.1016/j.ejmech.2019.06.071
Abuo-Rahma, G.D., Sarhan, H.A., and Gad, G.F., Bioorg. Med. Chem., 2009, vol. 17, p. 3879. https://doi.org/10.1016/j.bmc.2009.04.027
Foroumadi, A., Ghodsi, S., Emami, S., Najjari, S., Samadi, N., and Faramarzi, M.A., Bioorg. Med. Chem. Lett., 2006, vol. 16, p. 3499. https://doi.org/10.1016/j.bmcl.2006.03.103
Sriram, D., Aubry, A., Yogeeswari, P., and Fisher, L.M., Bioorg. Med. Chem. Lett., 2006, vol. 16, p. 2982. https://doi.org/10.1016/j.bmcl.2006.02.065
Rameshkumar, N., Ashokkumar, M., Subramanian, E.H., Ilavarasan, R., and Sridhar, S.K., Eur. J. Med. Chem., 2003, vol. 38, p. 1001. https://doi.org/10.1016/S0223-5234(03)00151-X
Akhtar, R., Noreen, R., Raza, Z., Rasul, A., and Zahoor, A.F., Russ. J. Org. Chem., 2022, vol. 58, p. 541. https://doi.org/10.1134/S107042802204011X
Arshad, M., Mohd Shoeb, K., and Shahab, A.A.N., Russ. J. Bioorg. Chem., 2021, vol. 47, p. 483. https://doi.org/10.1134/S1068162021020047
Patitungkho, S., Adsule, S., Dandawate, P., Padhye, S., Ahmad, A., and Sarkar, F.H., Bioorg. Med. Chem. Lett., 2011, vol. 21, p. 1802. https://doi.org/10.1016/j.bmcl.2011.01.061
Tejeswara Rao, A., Naresh Kumar, K., Venkanna, B., Srinubabu, M., Pal, M., and Jaya Shree, A., Lett. Drug Design Discove., 2018, vol. 15, p. 1087. https://doi.org/10.2174/1570180815666171229150032
Kini, D. and Ghate, M., Eur. J. Chem., 2011, vol. 8, p. 386.
Vazzana, I., Terranova, E., Mattioli, F., and Sparatore, F., Arkivoc, 2004, p. 364-374.
Tsyalkovsky, V.M., Kutsyk, R.V., Matiychuk, V.S., Obushak, N.D., and Klyufinskaya, T.I., Pharm. Chem. J., 2005, vol. 39, p. 245. https://doi.org/10.1007/s11094-005-0126-8
Terzioglu, N., Karali, N., Gursoy, A., Pannecouque, C., Leysen, P., Paeshuyse, J., Neyts, J., and De Clercq, E., Arkivoc, 2006, p. 109.
Ulusoy, N., Kiraz, M., Kucukbasmacl, O., and Monatshefte. Fur Chemie., 2002, vol. 133, p. 1305. https://doi.org/10.1007/s007060200108
Ali, M.M. and Hassan, S.A., Int. J. Cancer Res., 2007, vol. 3, p. 103.
Faidallah, H.M., Al-Saadi, M.S., Rostom, S.A.F., and Fahmy, H.T., Med. Chem. Res., 2007, vol. 16, p. 300. https://doi.org/10.1007/s00044-007-9033-8
Sriram, D., Yogeeswari, P., Senchani, G., and Banerjee, D., Bioorg Med Chem Lett., 2007, vol. 17, p. 2372. https://doi.org/10.1016/j.bmcl.2006.11.055
Raikwar, D.K., Srivastava, S.K., and Srivastava, S.D., J. Indian. Chem. Soc., 2008, vol. 85, p. 78.
Ravichandran, V., Mourya, V.K., Agrawal, R.K., Vishwadivyalala, H.S.G., and Digest, J., Nanomaterials Biostruct., 2008, vol. 3, p. 19.
Miller, M.J., Ji, C., and Miller, P.A., ACS Med. Chem. Lett., 2015, vol. 6, p. 707.
Vigorita, M.G., Ottana, R., Monforte, F., Maccari, R., Trovato, A., Monforte, M.T., and Taviano, M.F., Bioorg. Med. Chem. Lett., 2001, vol. 11, p. 2791. https://doi.org/10.1016/j.bmcl.2006.11.055
Sharma, P.C. and Jain, S., Acta Pol. Pharm., 2008, vol. 65, p. 551.
Shah, D.R., Lakum, H.P., and Chikhalia, K.H., Russ. J. Bioorg. Chem., 2015, vol. 41, p. 209.
Angelova, V.T., Pencheva, T., Buyukliev, R., Yovkova, E.K., Valkova, I., Momekov, G., and Vulcheva, V., Russ. J. Bioorg. Chem., 2021, vol. 47, p.122.
Srinivas, A., Nagaraj, A., and Sanjeeva Reddy, Ch., J. Heterocycl. Chem., 2008, vol. 45, p. 999. https://doi.org/10.1002/jhet.5570450409
Proudfoot, J.R., Bioorg. Med. Chem. Lett., 2002, vol. 12, p. 1647. https://doi.org/10.1016/S0960-894X(02)00244-5
Sahin, F., Karaman, I., Gulluce, M., Ogutcu, H., Xengul, M.S., Adıguzel, A., Ozturk, S., and Kotan, R., J. Ethnopharmacol., 2002, vol. 87, p. 61. https://doi.org/10.1016/S0378-8741(03)00110-7
Gulluce, M., Adıguzel, A., Ogutcu, H., Sengul, M., and Sahin, F., Phytother. Res., 2004, vol. 18, p. 208.
ACD/ChemSketch, version 2020.2.1, Advanced Chemistry Development, Inc., Toronto, ON, Canada, 2021. https://doi.org/10.1021/acschembio.1c00433
O’Boyle, N.M., Banck, M., and James, C.A., J. Cheminform., 2011, vol. 3, p. 33. https://doi.org/10.1186/1758-2946-3-33
Laponogov, I., Pan, X.S., Veselkov, D.A., Cirz, R.T., Wagman, A., Moser, H.E., Fisher, L.M., and Sanderson, M.R., Open Biol., 2016, vol. 6, p. 160157. https://doi.org/10.1098/rsob.160157
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., and Olson, A.J., J Comput. Chem., 2009, vol. 16, p. 2785. https://doi.org/10.1002/jcc.21256
Tejeswara Rao, A., Bhaskar, K., Naveen, P., Naveen, K., Kalyani, Ch., and Jaya Shree, A., Mol. Divers., 2022, vol. 26, p. 1581. https://doi.org/10.1007/s11030-021-10287-3
Zhao, Y., Abraham, M.H., Lee, J.A. Hersey, A., Luscombe, Ch.N., Beck, G., Sherborne, B., and Cooper, I., Pharm. Res., 2002, vol. 19, p. 1446. https://doi.org/10.1023/A:1020444330011
Ertl, P., Rohde, B., and Selzer, P., J. Med. Chem., 2000, vol. 43, p. 3714. https://doi.org/10.1021/jm000942e
ACKNOWLEDGMENTS
One of the authors (GSR) is thankful to Koneru Lakshmaiah Education Foundation for providing the required facilities and motivation for completion of the research work and special thanks to Dr. Saikrishna Balabadra, Assistant Professor, and the HOD, Dr. P. Venkat Reddy, SNIST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary information
Rights and permissions
About this article
Cite this article
Reddy, G.S., Rao, A.V., Keshavulu, M. et al. Design, Synthesis, and Biological Evaluation of Fluoroquinolones Linked to 4-Thiazolidinone Moieties as Potent Antimicrobial Agents: Docking Analysis. Russ J Gen Chem 92, 1749–1760 (2022). https://doi.org/10.1134/S1070363222090171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222090171