Abstract
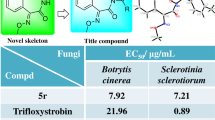
α-Methylene-γ-butyrolactone scaffold and benzofuran pharmacophore demonstrate a number of valuable medicinal properties. Based on bioactivity-guided mixed synthesis principle, we have designed and synthesized a series of new derivatives combining these two substructures. Antifungal activity of the products against some pathogenic fungi and cytotoxicity has been tested. QSAR has been performed. All зкщвгсеы demonstrate high activity against B. cinerea and G. graminis. Compounds with the 4-fluorophenyl group and compound connected with the cinnamic aldehyde structure demonstrate superior in vitro and in vivo activity. Results of SARs and QSAR studies exhibit that the lower electron density around the α-methylene-γ-butyrolactone backbone structure and smaller steric hindrance on the exocyclic carbon-carbon double bond are beneficial for antifungal activity. The results indicate benzbutyrolactone derivatives with α-methylene-γ-butyrolactone and benzofuran pharmacophore as highly active low-toxic compounds with the fungicide potential potential.




Similar content being viewed by others
REFERENCES
Brase, S., Encinas, A., Keck, J., and Nising, C.F., Chem. Rev., 2009, vol. 109, p. 3903. https://doi.org/10.1021/cr050001f
Dodds, P.N. and Rathjen, J.P., Nat. Rev. Genet., 2010, vol. 11, p. 539. https://doi.org/10.1038/nrg2812
Feng, J., Zhang, Y., Wang, J., and Zhang, X., Chin. J. Pesticide Sci., 2007, vol. 9, p. 185. https://doi.org/10.3321/j.issn:1008-7303.2007.02.016
Wang, D.L., Wang, L.Y., Wu, Y.L., Song, S., Feng, J.T., and Zhang, X., Eur. J. Med. Chem., 2017, vol. 130, p. 286. https://doi.org/10.1016/j.ejmech.2017.02.050
Feng, J.T., Ma, Z.Q., Li, J.H., He, J., Xu, H., and Zhang, X., Molecules, 2010, vol. 15, p. 6485. https://doi.org/10.3390/molecules15096485
Feng, J.T., Wang, H., Ren, S.X., He, J., Liu, Y., and Zhang, X., J. Agric. Food Chem, 2012, vol. 60, p. 3817. https://doi.org/10.1021/jf205123d
Wu, Y.L., Gao, Y.Q., Wang, D.L., Zhong, C.Q., Feng, J.T., Zhang, X., RSC Adv., 2017, vol. 7, p. 56496. https://doi.org/10.1039/C7RA12471F
Miyazawa, M., Shimabayashi, H., Hayashi, S., Hashimoto, S., Nakamura, S., Kosaka, H., and Kameoka, H., J. Agric. Food Chem., 2000, vol. 48, p. 5406. https://doi.org/10.1021/jf000346t
Han, C., Barrios, F.J., Mark, V.R., and David, A.C., Cheminform., 2010, vol. 5, p. 41. https://doi.org/10.1002/chin.201005184
Irakusne, L., Santiago, R., Javier, I., and Florenci, V., J. Org. Chem., 2007, vol. 72, p. 6614. https://doi.org/10.1021/jo0709955
Wu, Y.L., Wang, D.L., Guo, E.H., Song, S., Feng, J.T., and Zhang. X., Bioorg. Med. Chem. Lett., 2017, vol. 27, p. 1284. https://doi.org/10.1016/j.bmcl.2017.01.030
Shin, S.Y., Shin, M.C., Shin, J.S., Lee, K.T., and Lee, Y.S., Bioorg. Med. Chem. Lett., 2011, vol. 21, p. 4520. https://doi.org/10.1016/j.bmcl.2011.05.117
Cole, A.L., Hossain, S., Cole, A.M., and Phanstiel, O., Bioorg. Med. Chem., 2016, vol. 24, p. 2768. https://doi.org/10.1016/j.bmc.2016.04.045
Wu, Y.L., Wang, D.L., Gao, Y.Q., Feng, J.T., and Zhang. X., Molecules. 2016, vol. 21, p. 130. https://doi.org/10.3390/molecules21020130
Cho, A., Song, C.E., Lee, S.K., Shin, W.S., and Lim, E., J. Mater. Sci., 2016, vol. 51, p. 6770. https://doi.org/10.1007/s10853-016-9964-x
Sharma, P., Kumar, A., Upadhyay, S., Sahu, V., and Singh, J., Eur. J. Med. Chem., 2009, vol. 44, p. 251. https://doi.org/10.1016/j.ejmech.2008.02.016
Kalani, K., Yadav, D., Khan, F., Srivastava, S., and Suri, N., J. Mol. Model., 2012, vol. 18, p. 3389. https://doi.org/10.1007/s00894-011-1327-6
Funding
We greatly appreciate the funding support for this research provided by the Natural Science Foundation of Shaanxi Province (no. 2021JQ-842), Shengyong Zhang Academician Project of Shangluo University (no. 18YSZX005) and Introduction of Talent Research Start-up Fund of Shangluo University (no. 18SKY004). We also thank Northwest A&F University for the 1H NMR, 13C NMR, and HR-ESI-MS spectral data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Wu, YL., Wei, J., Meng, YF. et al. Synthesis, Antifungal Activity, and QSAR Studies of Benzbutyrolactone Derivatives Based on α-Methylene-γ-butyrolactone Scaffold. Russ J Gen Chem 92, 1085–1097 (2022). https://doi.org/10.1134/S1070363222060214
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222060214