Abstract
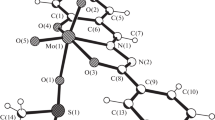
Two acetylhydrazones, namely benzoylacetone nicotinoylhydrazone (H2L1) and acetoacetanilide benzoylhydrazone (H2L2), and also the MoO2L1·MeOH solvate complex were synthesized and their structure was studied by single crystal X-ray diffraction and IR-spectroscopy methods. Molecules of two hydrazones crystallize in different enehydrazonic tautomeric forms: hydrazonic for H2L1 and enehydrazine-α-oxyazine for H2L2. In the both organic molecules, two planar six-membered aromatic cycles are connected by zigzag six- and seven-membered C–C–C–N–N(H)–C– and –N–C–C–C–C–N–N–C– chains, respectively. The both organic molecules are stabilized by N–H···O intermolecular hydrogen bonds (and also by N–H···O intramolecular hydrogen bond in the H2L2 structure). In the MoO2L1·MeOH solvate, the molybdenum atom has an octahedral coordination environment formed by two cis-O2(oxo) and tridentate (ONO)bis(chelate) (L2)2– ligands, and also a methanol molecule. Atoms N(L1) and O(MeOH) are in trans-position to O(oxo); two atoms O(L1)—in cis-position to O(oxo) and trans-position to each other.




Similar content being viewed by others
REFERENCES
Abdu, A.M., Al-Fakeh, M.S., Alhagri, I.A., Alhakimi, A.N., Saeed, S.E.-S., Shakdofa, A.M.E., and Shakdofa, M.M.E., J. Korean Chem. Soc., 2021, vol. 65, no. 2, p. 93. https://doi.org/10.5012/jkcs.2021.65.2.93
Busch, R., Carter, A.B., Konidaris, K.F., Kühne, I.A., González, R., Anson, Ch.E., and Powell, A.K., Chem. Eur. J., 2020, vol. 26, no. 51, p. 11835. https://doi.org/10.1002/chem.202001668
Kuriakose, D. and Kurup, M.R.P., Polyhedron, 2019, vol. 170, p. 749. https://doi.org/10.1016/j.poly.2019.06.041
Liang, M. and Zou, D.-H., Inorg. Nano-Met. Chem., 2017, vol. 47, no. 1, p. 110. https://doi.org/10.1080/15533174.2016.1149730
Majumder, S., Pasayat, S., Roy, S., Dash, S.P., Dhaka, S., Maurya, M.R., Reichelt, M., Reuter, H., Brzezinski, K., and Dinda, R., Inorg. Chim. Acta, 2018, vol. 469, p. 366. https://doi.org/10.1016/j.ica.2017.09.043
Kargar, H., Fallah-Mehrjardi, M., Behjatmanesh-Ardakani, R., Munawar, K.S., Ashfaq, M., and Tahir, M.N., Trans. Met. Chem., 2021, vol. 46, no. 6, p. 437. https://doi.org/10.1007/s11243-021-00460-w
Gamov, G.A. and Zavalishin, M.N., Russ. J. Inorg. Chem., 2021, vol. 66, no. 10, p. 1561. https://doi.org/10.1134/S0036023621100053
Zavalishin, M.N., Gamov, G.A., Khokhlova, A.Yu., Gashnikova, A.V., and Sharnin, V.A., Russ. J. Inorg. Chem., 2020, vol. 65, no. 1, p. 119. https://doi.org/10.1134/S0036023620010209
Sang, Y.-L., Zhang, X.-H., Lin, X.-S., Liu, Y.-H., and Liu, X.-Y., J. Coord. Chem., 2020, vol. 73, no. 1, p. 164. https://doi.org/10.1080/00958972.2019.1707192
Cvijanović, D., Pisk, J., Pavlović, G., Šišak-Jung, D., Matković-Calogović, D., Cindrić, M., Agustin, D., and Vrdoljak, V., New J. Chem., 2019, vol. 43, no. 4, p. 1791. https://doi.org/10.1039/c8nj04074e
Pisk, J., Rubčić, M., Kuzman, D., Cindrić, M., Agustin, D., and Vrdoljak, V., New J. Chem., 2019, vol. 43, no. 14, p. 5531. https://doi.org/10.1039/c9nj00229d
Mohan, B., Choudhary, M., Bharti, S., Jana, A., Das, N., Muhammad, S., Al-Sehemi, A.G., and Kumar, S., J. Mol. Struct., 2019, vol. 1190, p. 54. https://doi.org/10.1016/j.molstruc.2019.04.05
Sahani, A.J., Jayaram, R.V., and Burange, A.S., Mol. Сatal., 2018, vol. 450, p. 14. https://doi.org/10.1016/j.mcat.2018.02.028
Lakma, A., Hossain, S. M., Van Leusen, J., Kögerler, P., and Singh, A. K., Dalton Trans., 2019, vol. 48, no. 22, p. 7766. https://doi.org/10.1039/c9dt01041f
Hossain, S.M., Lakma, A., Pradhan, R.N., Demeshko, S., and Singh, A.K., Dalton Trans., 2017, vol. 46, no. 37, p. 12612. https://doi.org/10.1039/c7dt02433a
Maurya, M.R., Tomar, R., Rana, L., and Avecilla, F., Eur. J. Inorg. Chem., 2018, vol. 2018, no. 25, p. 2952. https://doi.org/10.1002/ejic.201800440
Maurya, M.R., Dhaka, S., and Avecilla, F., Polyhedron, 2015, vol. 96, p. 79. https://doi.org/10.1016/j.poly.2015.05.001
Moradi-Shoeili, Z., Boghae, D.M., Amini, M., Bagherzadeh, M., and Notas, B., Inorg. Chem. Commun., 2013, vol. 27, p. 26. https://doi.org/10.1016/j.inoche.2012.10.016
Pasayat, S., Dash, S.P., Roy, S., Dinda, R., Dhaka, S., Maurya, M.R., Kaminsky, W., Patil, Y.P., and Nethaji, М., Polyhedron, 2014, vol. 67, p. 1. https://doi.org/10.1016/j.poly.2013.08.055
Kurbah, S.D., Kumar, A., Syiemlieh, I., Asthana, M., and Lal, R.A., Inorg. Chem. Commun., 2017, vol. 86, p. 1. https://doi.org/10.1016/j.inoche.2017.09.016
Maurya, M.R., Rana, L., and Avecilla, F., Polyhedron, 2017, vol. 126, p. 60. https://doi.org/10.1016/j.poly.2017.01.006
Hu, X.-M., Xue, L.-W., Zhang, C.-X., and Zhao, G.-Q., Synth. React. Inorg. Met.-Org, Nano-Met. Chem., 2014, vol. 44, p. 713. https://doi.org/10.1080/15533174.2013.790435
Jaiswal, V., Gupta, S.R., Rastogi, R.B., Kumar, R., and Singh, V.P., J. Mater. Chem. A, 2015, vol. 3, no. 9, p. 5092. https://doi.org/10.1039/c4ta05663a
Rastogi, R.B., Maurya, J.L., and Jaiswal, V., Tribol. Trans., 2013, no. 56, p. 592. https://doi.org/10.1080/10402004.2012.748115
Pisk, J., Bilić, L., Đaković, M., Cvijanović, D., Damjanović, V., Lovrić, J., Rubčić, M., Vrdoljak, V., and Cindrić, M., Polyhedron, 2018, vol. 145, p. 70. https://doi.org/10.1016/j.poly.2018.02.003
Cordas, C.M. and Moura, J.J.G., Coord. Chem. Rev., 2019, vol. 394, p. 53. https://doi.org/10.1016/j.ccr.2019.05.005
Asha, T.M. and Kurup, M.R.P., Polyhedron, 2019, vol. 169, p. 151. https://doi.org/10.1016/j.poly.2019.04.045
Yusupov, V.G., Nasirdinov, S.D., Yakimovich, S.I., and Parpiev, N.Ya., Koord. Khim., 1984, vol. 10, no. 3, p. 387.
Kraudelt, H., Ludwig, E., Schilde, U., and Uhlemann, E., Z. Naturforsch., 1995, vol. 51b, p. 95.
Kargar, H., Kia, R., Froozandeh, F., Sadr, M.H., and Tahir, M.N., Acta Cryst. E, 2011, vol. 67, p. o209. https://doi.org/10.1107/S160053681005275X
Kargar, H., Kia, R., Moghadamm, M., and Tahir, M.N., Acta Cryst. E, 2011, vol. 67, p. o367. https://doi.org/10.1107/S1600536811000948
Paciorek, P., Szklarzewicz, J., Trzewik, B., Cież, D., Nitek, W., Hodorowicz, M., and Jurowska, A., J. Org. Chem., 2021, vol. 86, p. 1649. https://doi.org/10.1021/acs.joc.0c02451
Abramenko, V.L. and Sergienko, V.S., Russ. J. Inorg. Chem., 2009, vol. 54, no. 13, p. 2031. https://doi.org/10.1134/S0036028691.30014
Vrdoljak, V., Mandarić, M., Hrenar, T., Đilović, I., Pisk, J., Pavlović, G., Сindrić, M., and Agustin, D., Cryst. Growth Design, 2019, vol. 19, p. 3000. https://doi.org/10.1021/acs.cgd.9b00231
Sergienko, V.S., Abramenko, V.L., Churakov, A.V., Surazhskaya, M.D., Russ. J. Inorg. Chem., 2021, vol. 66, no. 12, p. 1854. https://doi.org/10.1134/S0036023621120159
Nandy, M., Shit, S., Rizzoli, C., Pilet, G., and Mitra, S., Polyhedron., 2015, vol. 88, p.63. https://doi.org/10.1016/j.poly.2014.12.017
Banße, W., Ludwig, E., Shilde, U., Uhlemann, E., Weller, F., and Lehmann, A., Z. Anorg. Allg. Chem., 1995, vol. 621, no. 7, p. 1275. https://doi.org/10.1002/zaac.19956210730
Sergienko, V.S., Abramenko, V.L., Minacheva, L.H., Porai-Koshits, M.A., and Saharova, V.G., Koord. Khim., 1993, vol.19, no. 1, p. 28.
Kargar, H., Porootan, P., Fallah-Mehrjardi, M., Behjatmanesh-Ardakani, R., Rudbayi, H.A., Munawar, K.S., Ashfaq, M., and Tahir, M.N., Inorg. Chim. Acta, 2021, vol. 523, p. 120414. https://doi.org/10.1016/j.ica.2021.120414
Sheldrick, G.M., SADABS, Program for Scaling and Correction of Area detector Data, University of Göttingen. Germany, 1997.
Sheldrick, G.M., Acta. Cryst. C, 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Funding
The work was carried out within the framework of the state assignment of the N.S. Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences in the field of fundamental scientific research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Sergienko, V.S., Abramenko, V.L., Churakov, A.V. et al. Synthesis and Structure of Dioxomolibdenum(VI) Complexes with Hydrazones of β-Dicarbonyl Compounds. Crystal Structures of Benzoylacetone Nicotinoylhydrazone (H2L1), Acetoacetanilide Benzoylhydrazone (H2L2), and MoO2L1·MeOH Solvate. Russ J Gen Chem 92, 1032–1039 (2022). https://doi.org/10.1134/S1070363222060147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222060147