Abstract
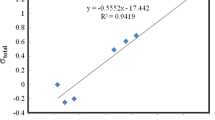
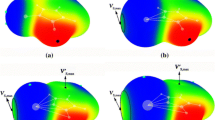
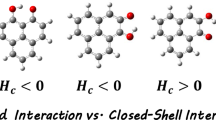
Quantum-chemical study (B3PW91/6-311G** and MP2/6-311G**) of structural and spectral criteria of the intramolecular coordination bond N→Si existence in [methoxy(methyl)silyl] derivatives of 8-mercaptoquinoline C9H6NSSi(OMe)nMe3–n (n = 1–3) has been carried out. Quantum topological analysis of electron distribution (AIM) has been performed to determine the physical nature of the hypervalent interaction. It has been found that the energy of the N→Si hypervalent bond depends on the number of the methoxy groups in the Si(OMe)3 fragment.





Similar content being viewed by others
REFERENCES
Kano, N., in Organosilicon Compounds Theory and Experiment (Synthesis), Lee, V.Ya., Ed., New York: Academic Press, 2017, p. 645. https://doi.org/10.1016/B978-0-12-801981-8.00011-3
Nikolin, A.A. and Negrebetsky, V.V., Russ. Chem. Rev., 2014, vol. 83, no. 9, p. 848. https://doi.org/10.1070/RC2014v083n09ABEH004385
Wagler, J., Böhme, U., and Kroke, E., Struct. Bond., 2014, vol. 155, p. 29. https://doi.org/10.1007/430_2013_118
Voronkov, M.G., Trofimova, O.M., Bolgova, Yu.I., and Chernov, N.F., Russ. Chem. Rev., 2007, vol. 76, no. 9, p. 825. https://doi.org/10.1070/RC2007v076n09ABEH003706
Reddy, A.C.S., Chen, Z., Hatanaka, T., Minami, T., and Hatanaka, Y., Organometallics, 2013, vol. 32, no. 13, p. 3575. https://doi.org/10.1021/om400017f
Li, Y., de Kock, C., Smith, P.J., Guzgay, H., Hendricks, D.T., Naran, K., Mizrahi, V., Warner, D.F., Chibale, K., and Smith, G.S., Organometallics, 2013, vol. 32, no. 1, p. 141. https://doi.org/10.1021/om300945c
Verkade, J.G., Coord. Chem. Rev., 1994, vol. 137, p. 233. https://doi.org/10.1016/0010-8545(94)03007-D
Pestunovich, V., Kirpichenko, S., and Voronkov, M., in The Chemistry of Organic Silicon Compounds, Rappoport, Z. and Apeloig, Y., Eds., Chichester: John Wiley & Sons Ltd., 1998, p. 1447. https://doi.org/10.1002/0470857250.ch24
Selina, A.A., Karlov, S.S., and Zaitseva, G.S., Chem. Heterocycl. Compd., 2006, vol. 42, no. 12, p. 1518. https://doi.org/10.1007/s10593-006-0278-9
Voronkov, M.G., Grebneva, E.A., Albanov, A.I., Zel’bst, E.A., Trofimova, O.M., Vasil’ev, A.D., Chernov, N.F., and Timofeeva, E.N., J. Organomet. Chem., 2014, vol. 768, p. 10. https://doi.org/10.1016/j.jorganchem.2014.05.025
Voronkov, M.G., Chernov, N.F., Albanov, A.I., Trofimova, O.M., Bolgova, Yu.I., Grebneva, E.A., Appl. Organometal. Chem., 2007, vol. 21, no. 7, p. 601. https://doi.org/10.1002/aoc.1271
Voronkov, M.G., Russ. Chem. Bull., 1991, vol. 40, no. 12, p. 2319. https://doi.org/10.1007/BF00959700
Voronkov, M.G. and Gubanova, L.I., Main Group Met. Chem., 1987, vol. 10, no. 4. P. 209.
Frolov, Yu.L. and Voronkov, M.G., J. Mol. Struct., 1990, vol. 217, p. 265. https://doi.org/10.1016/0022-2860(90)80367-S
Albanov, A.I., Voronkov, M.G., Gubanova, L.I., Larin, M.F., Liepin’sh, É.É., and Pestunovich, V.A., Russ. Chem. Bull., 1983, vol. 32, no. 10, p. 2165. https://doi.org/10.1007/BF00955799
Voronkov, M.G., Klyba, L.V., Vitkovskii, V.Yu., Gubanova, L.I., and Bochkarev, V.N., Russ. Chem. Bull., 1987, vol. 36, no. 7, p. 1392. https://doi.org/10.1007/BF01557508
Albanov, A.I., Gubanova, L.I., Larin, M.F., Pestunovich, V.A., and Voronkov, M.G., J. Organomet. Chem., 1983, vol. 244, no. 1, p. 5. https://doi.org/10.1016/S0022-328X(00)98629-7
Singh, G., Girdhar, S., and Promila, J., Applicable Chem., 2014, vol. 3, no. 5, p. 2066.
Belyaeva, V.V., Bolgova, Yu.I., Trofimova, O.M., and Albanov, A.I., Chem. Phys. Lett., 2019, vol. 715, p. 293. https://doi.org/10.1016/j.cplett.2018.11.050
Bondi, A., J. Phys. Chem., 1964, vol. 68, no. 3, p. 441. https://doi.org/10.1021/j100785a001
Lukevits, E. and Pudova, O.A., Chem. Heterocycl. Compd., 1996, vol. 32, nos. 11–12, p. 1381. https://doi.org/10.1007/BF01169969
Kummer, D., Halim, S.H.A., Kuhs, W., and Mattern, G., J. Organomet. Chem., 1993, vol. 446, no. 1–2, p. 51. https://doi.org/10.1016/0022-328X(93)80034-9
Macharashvili, A.A., Ovchinnikov, Yu.E., Struchkov, Yu.T., Sergeev, V.N., Pestunovich, S.V., and Baukov, Yu.I., Russ. Chem. Bull., 1993, vol. 42, no. 1, p. 173. https://doi.org/10.1007/BF00700003
Espinosa, E., Molins, E., and Lecomte, C., Chem. Phys. Lett., 1998, vol. 285, nos. 3–4, p. 170. https://doi.org/10.1016/S0009-2614(98)00036-0
Korlyukov, A.A., Lyssenko, K.A., and Antipin, M.Yu., Russ. Chem. Bull., 2002, vol. 51, no. 8, p. 1423. https://doi.org/10.1023/A:1021003919673
Cremer, D. and Kraka, E., Croat. Chem. Acta, 1984, vol. 57, no. 6, p. 1259.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A.Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Jr., Knox, E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannen berg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O.,, D.K. Malick, Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Mar tin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 03, Revision B.03, Gaussian, Inc., Pittsburgh, PA, 2003.
Bader, R.F.W., Chem. Rev., 1991, vol. 91, no. 5, p. 893. https://doi.org/10.1021/cr00005a013
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
To the 100th Anniversary of M.G. Voronkov
Rights and permissions
About this article
Cite this article
Belyaeva, V.V., Bolgova, Y.I. & Trofimova, O.M. Hypervalent Intramolecular N→Si Interaction in [Methoxy(methyl)silyl] Derivatives of 8-Mercaptoquinoline: Structural and Spectral Criteria. Russ J Gen Chem 92, 224–230 (2022). https://doi.org/10.1134/S1070363222020116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222020116