Abstract
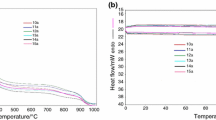
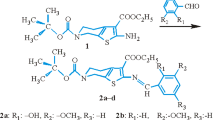
The reactions of one equimolar amount of monospirocyclotriphosphazenes bearing 2,2′-dioxybiphenyl with one, two, three and four equimolar amounts of potassium vanillinate were used to produce the vanillinato-substituted monospirocyclotriphosphazenes. The structures of new phosphazene derivatives were characterized by means of elemental analysis, IR and NMR (1H, 13C, and 31P) spectroscopic techniques. The molecular and crystal structures of mono and tetra vanillinato-substituted monospirocyclotriphosphazenes were examined using X-ray crystallography. The thermal degradation properties of all new compounds were determined using thermal gravimetric analysis (TGA) techniques.







Similar content being viewed by others
REFERENCES
Stewart, F.F., Organophosphorus Chemistry, Cambridge, UK: Royal Society of Chemistry, 2015, p. 397. https://doi.org/10.1039/9781782622765-00397
Okumuş, A., Bilge, S., Kılıç, Z., Öztürk, A., Hökelek, T., and Yılmaz, F., Spectrochim. Acta, Part A, 2010, vol. 76, p. 401. https://doi.org/10.1016/j.saa.2010.04.007
İlter, E.E., Çaylak, N., Işıklan, M., Asmafiliz, N., Kılıç, Z., and Hökelek, T., J. Mol. Struct., 2004, vol. 697, p. 119. https://doi.org/10.1016/j.molstruc.2004.03.043
Allen, C.W., Chem. Rev., 1991, vol. 91, p. 119. https://doi.org/10.1021/cr00002a002
Asmafiliz, N., İlter, E.E., Kılıç, Z., Hökelek, T., and Şahin, E., J. Chem. Sci., 2008, vol. 120, p. 363. https://doi.org/10.1007/s12039-008-0060-x
Huang, W.K., Yeh, J.T., Chen, K.J., and Chen, K.N., J. Appl. Polym. Sci., 2001, vol. 79, p. 662. https://doi.org/10.1002/1097-4628(20010124)79:4<662::AID-APP100>3.0.CO;2-T
Bolink, H.J., Barea, E., Costa, R.D., Coronado, E., Sudhakar, S., Zhen, C., and Sellinger, A., Org. Electron., 2008, vol. 9, p. 155. https://doi.org/10.1016/j.orgel.2007.10.005
Moriya, K., Suzuki, T., Yano, S., and Miyajima, S., J. Phys. Chem. B, 2001, vol. 105, p. 7920. https://doi.org/10.1021/jp004299j
Harrup, M.K., Gering, K.L., Rollins, H.W., Sazhin, S.V., Benson, M.T., Jamison, D.K., Michelbacher, C.J., and Luther, T.A., ECS Trans., 2012, vol. 41, p. 13. https://doi.org/10.1149/1.3703065
Allcock, H.R., Curr. Opin. Solid State Mater. Sci., 2006, vol. 10, p. 231. https://doi.org/10.1016/j.cossms.2007.06.001
Andrianov, A.K., DeCollibus, D.P., Gills, H.A., Kha, H.H., Marin, A., Prausnitz, M.R., Babiuk, L.A., Townsend, H., and Mutwiri, G., Proc. Natl. Acad. Sci., 2009, vol. 106, p. 18936. https://doi.org/10.1073/pnas.0908842106
Shcharbin, D., Dzmitruk, V., Shakhbazau, A., Goncharova, N., Seviaryn, I., Kosmacheva, S., Potapnev, M., Petziawiatr-Werbicka, E., Bryszewka, M., Talabaev, M., Chernov, A., Kulchitsky, V., Caminade, A.-M., and Majoral, J-P., Pharmaceutics, 2011, vol. 3, p. 458. https://doi.org/10.3390/pharmaceutics3030458
Morozowich, N.L., Weikel, A.L., Nichol, J.L., Chen, C., Nair, L.S., Laurencin, C.T., and Allcock, H.R., Macromolecules, 2011, vol. 44, p. 1355. https://doi.org/10.1021/ma1027406
Caminade, A.M., Chem. Commun., 2017, vol. 53, p. 9830. https://doi.org/10.1039/C7CC04949H
Asmafiliz, N., Kılıç, Z., Hayvalı, Z., Açık, L., Hökelek, T., Dal, H., and Öner, Y., Spectrochim. Acta, Part A, 2012, vol. 86, p. 214. https://doi.org/10.1016/j.saa.2011.10.027
Elmas, G., Okumuş, A., Koç, L.Y., Soltanzade, H., Kılıç, Z., Hökelek, T., Dal, H., Açık, L., Üstündağ, Z., Dündar, D., and Yavuz, M., Eur. J. Med. Chem., 2014, vol. 87, p. 662. https://doi.org/10.1016/j.ejmech.2014.10.005
Berberoğlu, İ., Asmafiliz, N., Kılıç, Z., Hökelek, T., Koç, L.Y., Açık, L., Türk, M., Soltanzade, H., and Dal, H., Inorg. Chim. Acta, 2016, vol. 446, p. 75. https://doi.org/10.1016/j.ica.2016.02.060
Asmafiliz, N., Civan, M., Uzunalioğlu, N., Özben, A., Kiliç, Z., Kayalak, H., Açik, L., and Hökelek, T., J. Chem. Sci., 2018, vol. 130, p. 152. https://doi.org/10.1002/aoc.4223
Tümer, Y., Asmafiliz, N., Zeyrek, T., Kılıç, Z., Açık, L., Çelik, S.P., Türk, M., Tunalı, B. Ç., Ünver, H., and Hökelek, T., New J. Chem., 2018, vol. 42, p. 1740. https://doi.org/10.1039/C7NJ03643D
Asmafiliz, N., Berberoğlu, İ., Özgür, M., Kılıç, Z., Kayalak, H., Açık, L., Türk, M., and Hökelek, T., Inorg. Chim. Acta, 2019, vol. 495, p. 118949. https://doi.org/10.1016/j.ica.2019.05.048
Uslu, A., and Yeşilot, S., Coord. Chem. Rev., 2015, vol. 291, p. 28. https://doi.org/10.1016/j.ccr.2015.01.012
Chandrasekhar, V., Pandian, B.M., and Azhakar, R., Polyhedron, 2008, vol. 27, p. 255. https://doi.org/10.1016/j.poly.2007.09.026
Maturana, R.G., Valenzuela, M.L., Schott, E., and Rojas-Poblete, M., Phys. Chem. Chem. Phys., 2017, vol. 19, p. 31479. https://doi.org/10.1039/C7CP06064E
Ainscough, E.W., Brodie, A.M., Chaplin, A.B., Derwahl, A., Harrison, J.A., and Otter, C.A., Analogues Inorg. Chem., 2007, vol. 46, p. 2575. https://doi.org/10.1021/ic062141t
Çil, E., and Arslan, M., Inorg. Chim. Acta, 2009, vol. 362, p. 1421. https://doi.org/10.1016/j.ica.2008.06.030
Ainscough, E.W., Brodie, A.M., Edwards, P.J.B., Jameson, G.B., Otter, C.A., and Kirk, S., Inorg. Chem., 2012, vol. 51, p. 10884. https://doi.org/10.1021/ic3013574
Koran, K., Özen, F., Biryan, F., and Görgülü, A.O., J. Mol. Struct., 2016, vol. 1105, p. 135. https://doi.org/10.1016/j.molstruc.2015.10.048
Şenkuytu, E., CBU J. Sci., 2018, vol. 14, p. 209. https://doi.org/10.18466/cbayarfbe.399162
Asmafiliz, N., Kılıç, Z., Öztürk, A., Süzen, Y., Hökelek, T., Açık, L., Çelik, Z.B., Koç, L. Y., Yola, M.L., and Üstündağ, Z., Phosphorus, Sulfur, Silicon Relat. Elem., 2013, vol. 188, p. 1723. https://doi.org/10.1080/10426507.2013.779273
Tümer, Y., Koç, L.Y., Asmafiliz, N., Kılıç, Z., Hökelek, T., Soltanzade, H., Açık, L., Yola, M.L., and Solak, A.O., J. Biol. Inorg. Chem., 2015, vol. 20, p. 165. https://doi.org/10.1007/s00775-014-1223-5
Tümer, Y., Asmafiliz, N., Arslan, G., Kılıç, Z., and Hökelek, T., J. Mol. Struct., 2019, vol. 1181, p. 235. https://doi.org/10.1016/j.molstruc.2018.12.090
Cremer, D. and Pople, J.A., J. Am. Chem. Soc., 1975, vol. 97, p. 1354. https://doi.org/10.1021/ja00839a011
Pidcock, E., Chem. Commun., 2005, vol. 27, p. 3457. https://doi.org/10.1039/B505236J
Tümer, Y., Asmafiliz, N., Kılıç, Z., Aydın, B., Açık, L., and Hökelek, T., J. Mol. Struct., 2018, vol. 1173, p. 885. https://doi.org/10.1016/j.molstruc.2018.07.050
Bullen, G., J. Chem. Soc. A, 1971, vol. 56, p. 1450.
Levchik, S.V., Camıno, G., Luda, M.P., Costra, L., Lindsay, A., and Stevenson, D., J. Appl. Polym. Sci., 1998, vol. 67, p. 461. https://doi.org/10.1002/(SICI)1097-4628(19980118)67:3<461::AID-APP9>3.0.CO;2-K
Akbaş, H., Okumuş, A., Karadağ, A., Kılıç, Z., Hökelek, T., Koç, L.Y., Açik, L., Aydin, B., and Türk, M., J. Therm. Anal. Calorim., 2015, vol. 123, p. 1627. https://doi.org/10.1007/s10973-015-5001-6
Okumuş, A., Akbaş, H., Karadağ, A., Aydın, A., Kılıç, Z., and Hökelek, T., ChemistrySelect, 2017, vol. 2, p. 4988. https://doi.org/10.1002/slct.201700497
Tümer, Y., Batı, H., Çalışkan, N., Yüksektepe, Ç., and Büyükgüngör, O., Z. Anorg. Allg. Chem., 2008, vol. 634, p. 597. https://doi.org/10.1002/zaac.200700389
Bruker Program 1D WIN-NMR (Release 6.0) and 2D WIN-NMR (Release 6.1)
Bruker 2005 SADABS Bruker AXS Inc. Madison Wisconsin USA
Sheldrick, G.M., Acta Crystallogr., Sect A, 2008, vol. 64, p. 112. https://doi.org/10.1107/S0108767307043930
Farrugia, L.J., J. Appl. Crystallogr., 1997, vol. 30, p. 565. https://doi.org/10.1107/S0021889897003117
Allcock, H.R., Stein, M.T., and Stanko, J.A., J. Am. Chem. Soc., 1971, vol. 93, p. 3173.
Funding
This work was financially supported by “Karabük University-BAP” Grant no. KBÜBAP-17-YL-440.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Tümer, Y., Özdemir, S.Z. Vanillinato-Substituted Monospirocyclotriphosphazenes: Synthesis, Spectroscopic and Crystallographic Characterizations, and Thermal Properties. Russ J Gen Chem 91, 2554–2563 (2021). https://doi.org/10.1134/S1070363221120276
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221120276