Abstract
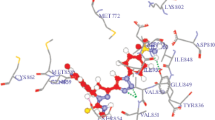
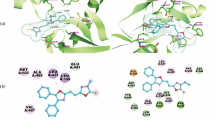
This study is devoted to the efficient and practical synthesis of a novel series of pyrido[4,3-d]pyrimidine derivatives attached to 1,2,3-triazole ring and lipophilic terminal fractions. Structure of the newly synthesized compounds is well characterized by various spectroscopic methods. An in vitro MTT cytotoxicity assay has been used to compare cytotoxic effects of the synthesized compounds on MCF-7, HeLa, and A-549. The kinase inhibitory assay against tyrosine kinase EGFR has been performed for the potent compounds and the results are supporting their in vitro anticancer activity. Further study of their binding affinity has been performed by molecular docking with the EGFR site. The molecular docking and cytotoxic tests results correlate well.


Similar content being viewed by others
REFERENCES
Bozorov, K., Zhao, J., and Aisa, H.A., Bioorg. Med. Chem., 2019, vol. 27, p. 3511. https://doi.org/10.1016/j.bmc.2019.07.005
Qingyun, R., Yong-Ju, L., Hongwu, H., Liwu, F., and Yucheng, G., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 6713. https://doi.org/10.1016/j.bmcl.2009.09.117
Narsimha, S., Kumar, N.S., Swamy, B.K., Reddy, N.V., Althaf Hussain, S.K., and Rao, M.S., Bioorg. Med. Chem. Lett., 2016, vol. 26, p. 1639. https://doi.org/10.1016/j.bmcl.2016.01.055
Narsimha, S., Sathesh, K.N., Savitha, J.T., Ravinder, M., Srinivasa, R.M., and Vasudeva, R.N., J. Heterocyclic. Chem., 2020, vol. 57(4), p. 1655. https://doi.org/10.1002/jhet.3890
Kumara, S.B., Narsimha, S., Ranjith, K.T., Narsimha, R.Y., and Vasudeva, R.N., ChemistrySelect., 2017, vol. 2(14), p. 4001. https://doi.org/10.1002/slct.201700524
Kumara, S.B., Narsimha, S., Ranjith, K.T., Narsimha, R.Y., and Vasudeva, R.N., ChemistrySelect., 2017, vol. 2(29), p. 9595. https://doi.org/10.1002/slct.201701902
Narsimha, S., Kumara, S.B., Yellu, N.R., and Vasudeva, R.N., Chem. Heterocycl. Compd., 2018, vol. 54, p. 1161. https://doi.org/10.1007/s10593-019-02408-6
Zhi, X., Shi-Jia, Z., and Yi, L., Eur. J. Med. Chem., 2019, vol. 183, p. 111700. https://doi.org/10.1016/j.ejmech.2019.111700
Ramya, S.E., Satheesh, K.N., Ravinder, M., Vasudeva, R.N., and Narsimha, S., Russ. J. Bioorg. Chem., 2021, vol. 47, p. 896. https://doi.org/10.1134/S1068162021040208
Venkatesh, E., Narsimha, S., Kumar, N. S., and Reddy, N.V., Russ. J. Gen. Chem., 2020, vol. 90, p. 2444. https://doi.org/10.1134/S1070363220120361
Khaled, M.E., and Başak, D.M., RSC Adv., 2016, vol. 6, p. 71827. https://doi.org/10.1039/C6RA12364C
Tornoe, C.W., Christensen, C., and Meldal, M., J. Org. Chem., 2002, vol. 67, p. 3057. https://doi.org/10.1021/jo011148j
Ramesh, G., Ratni, S., Krishnaiah, V., Sarika, S., Narsimha, S., Rajitha, B., and Anil, K.S., ChemistrySelect, 2018, vol. 3, p. 1424. https://doi.org/10.1002/slct.201702971
Ravikumar, R.S., Satheesh, K.N., Rajkumar, N., Narsimha, S., Prasad, G., Ravinder, M., and Narasimha, S.T., ChemistrySelect., 2021, vol. 6, p. 7670. https://doi.org/10.1002/slct.202101820
Roskoski, R.Jr., Pharmacol. Res., 2014, vol. 79, p. 34. https://doi.org/10.1016/j.phrs.2013.11.002
Sebastian, J., Richards, R.G., Walker, M.P., Wiesen, J.F., Werb, Z., Derynck, R., Hom, Y.K., Cunha, G.R., and DiAugustine, R.P., Cell Growth Differ.,1998, vol. 9, p. 777.
Walker, F., Abramowitz, L., Benabderrahmane, D., Duval, X., Descatoire, V., Henin, D., Lehy, T., and Aparicio, T., Hum. Pathol., 2009, vol. 40, p. 1517. https://doi.org/10.1016/j.humpath.2009.05.010
McBryan, J., Howlin, J., Napoletano, S., and Martin, F., J. Mammary Gland Biol. Neoplasia., 2008, vol. 13, p. 159. https://doi.org/10.1007/s10911-008-9075-7
Park, J.H. and Lemmon, M.A., Biochem. J., 2012, vol. 448, p. 417. https://doi.org/10.1042/BJ20121513
ACKNOWLEDGMENTS
The authors are thankful to the head, Department of Biotechnology, Chaitanaya Deemed to be University, Warangal, for providing data of biological activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Sreerama, R., Kumar, N.M., Nukala, S.K. et al. Synthesis and Biological Evaluation of Novel 1,2,3-Triazole Based Pyrido[4,3-d]pyrimidines as Potent Anticancer and EGFR Inhibitors. Russ J Gen Chem 91, 2515–2521 (2021). https://doi.org/10.1134/S1070363221120227
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221120227