Abstract
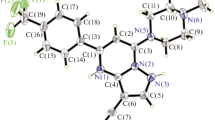
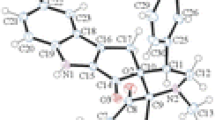
N-{4-[(6-bromopyrido[2,3-d]pyrimidin-4-yl)oxy]phenyl}morpholine-4-carboxamide has been synthesized as a derivative of pyrido[2,3-d]pyrimidine that demonstrates antitumor, antibacterial, anti-inflammatory, and antimicrobial activities. Synthesis of the target compound based on 2-aminonicotinic acid as the starting material has included its esterification, bromination, cyclization, and substitution reactions. Structure of the product is confirmed by 1H and 13C NMR, FT-IR, and single-crystal X-ray diffraction (XRD). The optimized structure, electrostatic potential and frontier molecular orbitals (FMO) of the compound have been approached by DFT calculations. The compound demonstrates antiproliferative activity on A375 cells.








Similar content being viewed by others
REFERENCES
Fares, M., Hadi, S.R.A.E., Eladwy R.A., Shoun, A.A., Abdel-Aziz, M.M., Eldehna, W.M., Abdel-Aziz, and Keller, H.A.P.A., Org. Biomol. Chem., 2018, vol. 16, p. 3389. https://doi.org/10.1039/c8ob00627j
Ribble, W., Hill, W.E., Ochsner, U.A., Jarvis, T.C., Guiles, J.W., Janjic, N., and Bullard, J.M., Antimicrob. Agent. Chemother., 2010, vol. 54, p. 4648. https://doi.org/10.1128/aac.00638-10
Rajesh, S.M., Kumar, R.S., Libertsen, L.A., Perumal, S., Yogeeswari, P., and Sriram, D., Bioorg. Med. Chem. Lett., 2011, vol. 21, p. 3012. https://doi.org/10.1016/j.bmcl.2011.03.045
Mohamed, N.R., Abdelhalim, M.M., Khadrawy, Y.A., and Elmegeed, G.A., Steroids., 2012, vol. 77, p. 1469. https://doi.org/10.1016/j.steroids.2012.09.001
Nam, G., Yoon, C.M., Kim, E., Rhee, C.K., Kim, J.H., Shin, J.H., and Kim, S.H., Bioorg. Med. Chem. Lett., 2001, vol. 11, p. 611. https://doi.org/10.1016/s0960-894x(00)00681-8
Nakayama, K., Kawato, H., Watanabe, J., Ohtsuka, M., Yoshida, K., Yokomizo, Y., Sakamoto, A., Kuru, N., Ohta, T., Hoshino, K., Yoshida, K., Ishida, H., Cho, A., Palme, M.H., Zhang, J.Z., Lee, V.J., and Watkins, W.J., Bioorg. Med. Chem. Lett., 2004, vol. 14, p. 475. https://doi.org/10.1016/j.bmcl.2003.10.060
Farghaly, T.A. and Hassaneen, H.M.E., Arch. Pharm. Res., 2013, vol. 36, p. 564. https://doi.org/10.1007/s12272-013-0045-2
Quintela, J.M., Peinador, C., Botana, L., Estévez, M., and Riguera, R., Bioorg. Med. Chem., 1997, vol. 5, p. 1543. https://doi.org/10.1016/s0968-0896(97)00108-9
El-Subbagh, H.I., Abu-Zaid, S.M., Mahran, M.A., Badria, F.A., and Al-Obaid, A.M., J. Med. Chem., 2000, vol. 43, p. 2915. https://doi.org/10.1021/jm000038m
Bennett, L.R., Blankley, C.J., Fleming, R.W., Smith, R.D., and Tessman, D.K., J. Med. Chem., 1981, vol. 24, p. 382. https://doi.org/10.1021/jm00136a006
Gfesser, G.A., Bayburt, E.K., Cowart, M., DiDomenico, S., Gomtsyan, A., Lee, C.H., Stewart, A.O., Jarvis, M.F., Kowaluk, E.A., and Bhagwat, S.S., Eur. J. Med. Chem., 2003, vol. 38, p. 245. https://doi.org/10.1016/s0223-5234(03)00019-9
Chen, D., Chen, Y., Yang, D., Zheng, Z., and Zhou, Z., J. Heterocyclic Chem., 2021, vol. 58, p. 1628. https://doi.org/10.1002/jhet.4287
Zhao, J.S., Jin, P., Xi, N., Wei, D.D., Li, J., Wei, D., and Hu, C.H., J. Struct. Chem., 2017, vol. 36, p. 937. https://doi.org/10.14102/j.cnki.0254-5861.2011-1437
Dereli, Ö., Opt. Spectrosc., 2016, vol. 120, p. 690. https://doi.org/10.1134/s0030400x16050222
Brintzinger, H.H., Prosenc, M.H., Schaper, Weeber, F.A., and Wieser, U., J. Mol. Struct., 1999, vol. 485, p. 409. https://doi.org/10.1016/s0022-2860(99)00184-2
Huang, N., Kalyanaraman, C., Bernacki, K., and Jacobson, M.P., Phys. Chem. Chem. Phys., 2006, vol. 8, p. 5166. https://doi.org/10.1039/b608269f
Sheldrick, G.M., Acta Cryst. (A), 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053273314026370
Im, G.-Y.J., Bronner, S.M., Goetz, A.E., Paton, R.S., Cheong, P.H.-Y., Houk, K.N., and Garg, N.K., J. Am. Chem. Soc., 2010, vol. 132, p. 17933. https://doi.org/10.1021/ja1086485
Andersson, M.P. and Uvda, P., J. Phys. Chem., 2005, vol. 109, p. 2937. https://doi.org/10.1021/jp045733a
Krishnakumar, V. and John, X.R., Spectrochim. Acta, Part A, 2006, vol. 63, p. 454. https://doi.org/10.1016/j.saa.2005.05.031
Mague, J.T., Mohamed, S.K., and Akkurt, M., Acta Cryst. (E), 2015, vol. 71, p. o1005. https://doi.org/10.1107/S2056989015022495
Sheldrick, G. M., Acta Cryst. (C), 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Rahmani, R., Boukabcha, N., Chouaih, A., Hamzaoui, F., and Goumri Said, S., J. Mol. Struct., 2018, vol. 1155, p. 484. https://doi.org/10.1016/j.molstruc.2017.11.033
ACKNOWLEDGMENTS
This work has been awarded the Guizhou Provincial Natural Science Foundation ([2020]1Y393).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary information
Rights and permissions
About this article
Cite this article
Deng, L., Sun, H., Hu, W. et al. Synthesis, Crystal Structure, and DFT Study of a New Derivative of Pyrido[2,3-d]pyrimidine. Russ J Gen Chem 91, 2489–2496 (2021). https://doi.org/10.1134/S1070363221120197
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221120197