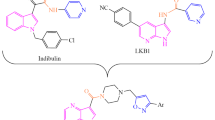
Abstract
Herein, we report a series of 1,2,3-triazole combined with quinazoline hybrid heterocyclic compounds by 1,3-dipolar cycloaddition reaction catalyzed by Cu(I). This reaction has been carried out between quinazoline based alkyne and aryl/benzyl azides under optimized conditions. Modification of 4-hydroxyquinazoline by propargyl bromide in presence of potassium carbonate gives a corresponding alkyne. Structure of the synthesized compounds has been characterized by IR, 1H, and 13C NMR, ESI-Mass, and HRMS spectra. The products have been tested for anticancer activity against C6 glioma cell lines and characterized by moderate cytotoxic activity.



Similar content being viewed by others
REFERENCES
Ajani, O., Audu, O., Aderohunmu, D., Owolabi, F., and Olomieja., A., Am. J. Drug Discov. Dev., 2017, vol. 7, p. 1. https://doi.org/10.3923/ajdd.2017.1.24
Gupta, T., Rohilla, A., Pathak, A., Akhtar, M.J., Haider, M.R., and Yar, M.S., Synth. Commun., 2018, vol. 48, p. 1099. https://doi.org/10.1080/00397911.2018.1431282
Alafeefy, A.M., Kadi, A.A., Al-Deeb, O.A., El-Tahir, K.E., and Al-Jaber, N.A., Eur. J. Med. Chem., 2010, vol. 45, p. 4947. https://doi.org/10.1016/j.ejmech.2010.07.067
Chandregowda, V., Kush, A.K., and Chandrasekara Reddy, G., Eur. J. Med. Chem., 2009, vol. 44, p. 3046. https://doi.org/10.1016/j.ejmech.2008.07.023
El-Azab, A.S., Al-Omar, M.A., Alaa, A.-M., AbdelAziz, N.I., Magda, A.-A., Aleisa, A.M., Sayed-Ahmed, M.M., and Abdel-Hamide, S.G., Eur. J. Med. Chem., 2010, vol. 45, p. 4188. https://doi.org/10.1016/j.ejmech.2010.06.013
Zhang, L., Fan, C., Guo, Z., Li, Y., Zhao, S., Yang, S., Yang, Y., Zhu, J., and Lin, D., Eur. J. Med. Chem., 2013,vol. 69, p. 833. https://doi.org/10.1016/j.ejmech.2013.09.032
Kamal, A., Prabhakar, S., Ramaiah, M.J., Reddy, P.V., Reddy, C.R., Mallareddy, A., Shankaraiah, N., Reddy, T.L.N., Pushpavalli, S., and Pal-Bhadra, M., Eur. J. Med. Chem., 2011, vol. 46, p. 3820. https://doi.org/10.1016/j.ejmech.2011.05.050
Shafi, S., Alam, M.M., Mulakayala, N., Mulakayala, C., Vanaja, G., Kalle, A.M., Pallu, R., and Alam, M., Eur. J. Med. Chem., 2012, vol. 49, p. 324. https://doi.org/10.1016/j.ejmech.2012.01.032
Tahghighi, A., Razmi, S., Mahdavi, M., Foroumadi, P., Ardestani, S.K., Emami, S., Kobarfard, F., Dastmalchi, S., Shafiee, A., and Foroumadi, A., Eur. J. Med. Chem., 2012, vol. 50, p. 124. https://doi.org/10.1016/j.ejmech.2012.01.046
da Silva, F.d.C., de Souza, M.C.B.V., Frugulhetti, I.I.P., Castro, H.C., Souza, S.L.d.O., de Souza, T.M.L., Rodrigues, D.Q., Souza, A.M.T., Abreu, P.A., Passamani, F., Rodrigues, C.R., and Ferreira, V.F., Eur. J. Med. Chem., 2009, vol. 44, p. 373. https://doi.org/10.1016/j.ejmech.2008.02.047
Chan, D.C.M., Laughton, C.A., Queener, S.F., and Stevens, M.F.G., Bioorg. Med. Chem., 2002, vol. 10, p. 3001. https://doi.org/10.1016/S0968-0896(02)00128-1
Hassan, H.M.D.I., Khan, S.A., Rehan, M., Sakkaf, K., and Gauthaman, K., Med. Chem. Res., 2019, vol. 28, p. 1766. https://doi.org/10.1007/s00044-019-02413-6
Banerji, B., Chandrasekhar, K., Sreenath, K., Roy, S., Nag, S., and Saha, K.D., ACS Omega., 2018, vol. 3, p. 16134. https://doi.org/10.1021/acsomega.8b01960
Brahmaiah, D.A.K.D.B., Aparna, P., Sampath Kumar, N., Solhi, H., Le Guevel, R., Baratte, B., Ruchaud, S., Bach, S., Mosset, P., and Gree, R., Arkivoc., 2019, vol. 5, p. 96. https://doi.org/10.24820/ark.5550190.p010.859
Brahmaiah, D., Kanaka Durga Bhavani, A., Aparna, P., Sampath Kumar, N., Solhi, H., Le Guevel, R., Baratte, B., Ruchaud, S., Bach, S., Singh Jadav, S., Raji Reddy, C., Roisnel, T., Mosset, P., Levoin, N., and Grée, R., Bioorg. Med. Chem., 2021, vol. 31, p. 115962. https://doi.org/10.1016/j.bmc.2020.115962
Anand, N., Singh, P., Sharma, A., Tiwari, S., Singh, V., Singh, D.K., Srivastava, K.K., Singh, B.N., and Tripathi, R.P., Bioorg. Med. Chem., 2012, vol. 20, p. 5150. https://doi.org/10.1016/j.bmc.2012.07.009
Genady, A.R., Eur. J. Med. Chem., 2009, vol. 44, p. 409. https://doi.org/10.1016/j.ejmech.2008.02.037
Vodnala, S., Bhavani, A.K.D., Kamutam, R., Naidu, V.G.M., Promila, and Prabhakar, C., Bioorg. Med. Chem. Lett., 2016, vol. 26, p. 3973. https://doi.org/10.1016/j.bmcl.2016.07.003
Carmichael, J., DeGraff, W.G., Gazdar, A.F., Minna, J.D., and Mitchell, J.B., Cancer Res., 1987, vol. 47, p. 936.
ACKNOWLEDGMENTS
VS is grateful to Department of Chemistry, Osmania University for providing research facilities. The authors express their gratitude and thanks to the CFRD, Osmania University, for providing NMR facility. The authors are thankful to OU SAP, DST-PURSE-II, DST-FIST programme for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Vodnala, S., Bhavani, A.K.D., Pagilla, S. et al. Synthesis and Cytotoxic Studies of Quinazoline-Triazole Hybrid Aza Heterocycles. Russ J Gen Chem 91, 2304–2310 (2021). https://doi.org/10.1134/S1070363221110189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221110189