Abstract
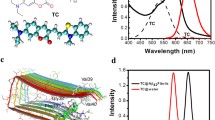
A triamine aryl pyrazole-based NIR fluorescent probe with good water solubility has been developed for detecting and imaging Aβ aggregates in Alzheimer’s disease. In vitro studies have demonstrated that the probe high affinity to Aβ aggregates with an increase of fluorescence intensity have been due to the intramolecular charge transfer effect. The probes can specifically image Aβ plaques in brain sections of triple transgenic AD mice.





Similar content being viewed by others
REFERENCES
Roberson, E.D. and Mucke, L., Science, 2006, vol. 314, no. 5800, p. 781. https://doi.org/10.1126/science.1132813
Kim, J.H., Lee, J., Lee, S., and Cho, E.J., Appl. Bio. Chem., 2016, vol. 59, p. 721. https://doi.org/10.1007/s13765-016-0217-0
Mathis, C.A., Wang, Y., and Klunk, W.E., Curr. Pharm. Design, 2004, vol. 10, no. 13, p. 1469. https://doi.org/10.2174/1381612043384772
Nordberg, A., The Lancet Neurology, 2004, vol. 3, no. 9, p. 519. https://doi.org/10.1016/S1474-4422(04)00853-1
Selkoe, D.J., Nature Biotechnol., 2000, vol. 18, p. 823. https://doi.org/10.1038/78422
Zhu, L., Ploessl, K., and Kung, H.F., Chem. Soc. Rev., 2014, vol. 43, no. 19, p. 6683. https://doi.org/10.1039/C3CS60430F
Xu, Z., Chen, J., Hu, L. L., Tan, Y., Liu, S. H., and Yin, J., Chin. Chem. Lett., 2017, vol. 28, no. 10, p. 1935. https://doi.org/10.1016/j.cclet.2017.07.018
Huang, H., Liu, M., Wan, Q., Jiang, R., Xu, D., Huang, Q., Wen, Y., Deng, F., Zhang, X., and Wei, Y., Mater. Sci. Eng. C, 2018, vol. 91, p. 201. https://doi.org/10.1016/j.msec.2018.05.015
Weissleder, R., and Mahmood, U., Radiology, 2001, vol. 219, no. 2, p. 316. https://doi.org/10.1148/radiology.219.2.r01ma19316
Liu, K., Guo, T., Chojnacki, J., Lee, H., Wang, X., Siedlak, S., Rao, W., Zhu, X., and Zhang, S., ACS Chem. Neurosci., 2012, vol. 3, no. 2, p. 141. https://doi.org/10.1021/cn200122j
Staderini, M., Martin, M.A., Bolognesi, M.L., and Carlos Menendez, J., Chem. Soc. Rev., 2015, vol. 44, p. 1807. https://doi.org/10.1039/C4CS00337C
Fu, H., Cui, M., Zhao, L., Tu, P., Zhou, K., Dai, J., and Liu, B., J. Med. Chem., 2015, vol. 58, no. 17, p. 6972. https://doi.org/10.1021/acs.jmedchem.5b00861
Ono, M., Watanabe, H., Kimura, H., and Saji, H., ACS Chem. Neurosci., 2012, vol. 3, no. 4, p. 319. https://doi.org/10.1021/cn3000058
Watanabe, H., Ono, M., and Saji, H., Chem. Commun., 2015, vol. 51, p. 17124. https://doi.org/10.1039/C5CC06628J
Cao, K., Farahi, M., Dakanali, M., Chang, W.M., Sigurdson, C.J., Theodorakis, E.A., and Yang, J., J. Am. Chem. Soc., 2012, vol. 134, no. 42, p. 17338. https://doi.org/10.1021/ja3063698
Li, Y., Kan, W., Zhou, K., Guo, W., Dai, B., Yi, L., Dai, J., and Cui, M., Chem. Commun., 2018, vol. 54, p. 8717. https://doi.org/10.1039/C8CC05259J
Lv, G., Sun, A., Wei, P., Zhang, N., Lan, H., and Yi, T., Chem. Commun., 2016, vol. 52, p. 8865. https://doi.org/10.1039/C6CC02741E
Lv, G., Cui, B., Lan, H., Wen, Y., Sun, A., and Yi, T., Chem. Commun., 2015, vol. 51, p. 125. https://doi.org/10.1039/C4CC07656G
Wang, D., Su, H., Kwok, R.T.K., Hu, X., Zou, H., Luo, Q., Lee, M.S., Xu, W., Lam, J.W.Y., and Tang, B.Z., Chem. Sci., 2018, vol. 9, p. 3685. https://doi.org/10.1039/C7SC04963C
Kopka, K., Faust, A., Keul, P., Wagner, S., Breyholz, H.J., Holtke, C., Schober, O., Schafers, M., and Levkau, B., J. Med. Chem. 2006, vol. 49, no. 23, p. 6704. https://doi.org/10.1021/jm051217c
Wakamiya, A., Taniguchi, T., and Yamaguchi, S., Angew. Chem. Int. Ed., 2006, vol. 118, no.19, p. 3242. https://doi.org/10.1002/ange.200504391
Chang, W.M., Dakanali, M., Capule, C.C., Sigurdson, C.J., Yang, J., and Teodorakis, E.A., ACS Chemical Neurosci., 2011, vol. 2, no. 5, p. 249. https://doi.org/10.1021/cn200018v
Antonello, S. and Maran, F., Chem. Soc. Rev., 2005, vol. 34, p. 418. https://doi.org/10.1039/B300085K
Prusiner, S.B., Scott, M.R., DeArmond, S.J., and Cohen, F.E., Cell, 1998, vol. 93, no. 3, p. 337. https://doi.org/10.1016/S0092-8674(00)81163-0
Li, Y.H., Xu, D., Ho, S.L., Li, H.W., and Yang, R.H., Biomaterials, 2016, vol. 94, p. 84. https://doi.org/10.1016/j.biomaterials.2016.03.047
Funding
This research was financially supported by the Natural Science Foundation of Anhui Province (no. 1808085MB47), Department of Education Committee of Anhui Province and the Center for Anhui Province Engineering of Modern Chinese traditional medicine (no. KJ2017A291).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, J.F., Zhou, Y., Xu, G.Y. et al. A Triphenylamine Derivative-based Fluorescent Probe with Good Water Solubility for Targeting Aβ Plaques in Alzheimer’s Disease. Russ J Gen Chem 91, 1748–1756 (2021). https://doi.org/10.1134/S1070363221090218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221090218